Cathode Research
Polyanionic Materials

Electrochemical Properties of Nanostructured Copper Hydroxysulfate Mineral Brochantite upon Reaction with Lithium
Ran Zhao, Ting Yang, Michael A. Miller, Candace K. Chan
Nano Lett. 2013, 13, 12, 6055–6063
Cu-containing conversion electrodes have received much study as high capacity electrodes for lithium-ion batteries, but many suffer from poor reversibility. The electrochemical properties of the copper hydroxysulfate compound, Cu4(OH)6SO4, more commonly known as the mineral brochantite and as a patina constituent on the Statue of Liberty, were investigated. Nanostructured brochantite was synthesized using precipitation and microwave-assisted hydrothermal reactions and evaluated in half-cells with Li metal counter electrodes. Reversible capacities >400 mAh/g corresponding to the 2 electron reduction of Cu2+ and discharge potential of 1.8 V versus Li/Li+ were observed in brochantite with a nanoplate morphology. Detailed characterization using X-ray diffraction, scanning and transmission electron microscopy, and X-ray photoelectron spectroscopy was performed to better understand the conversion process.
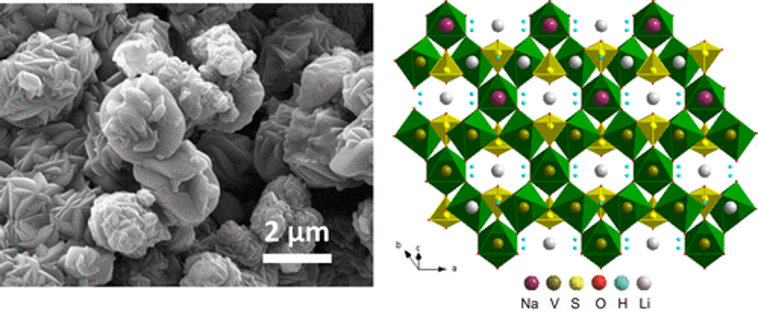
Synthesis of Jarosite and Vanadium Jarosite Analogues Using Microwave Hydrothermal Reaction and Evaluation of Composition-Dependent Electrochemical Properties
Ran Zhao, Ying Li, Candace K. Chan
J. Phys. Chem. C 2016, 120, 18, 9702–9712
Polyanion compounds are attractive as electrode materials for Li-ion batteries due to their low cost, good stability, and safety. Here we used microwave hydrothermal synthesis to prepare a series of jarosite compounds, AM3(SO4)2(OH)6, where A = K, Na and M = Fe, V. Both morphology and electrochemical properties of the materials in Li half-cells showed a composition dependence. At potentials >1.5 V vs Li/Li+, an insertion-type reaction was observed in Na,Fe-jarosite but not in K,Fe-jarosite, likely due to the presence of intercalated H3O+. Reversible insertion-type reactions were observed in both vanadium jarosites between 1–4 V with capacities around 40–60 mAh/g. Below 1 V vs Li/Li+, all four jarosite compounds underwent conversion reactions with capacities ∼500 mAh/g observed for the Fe-jarosites. X-ray diffraction showed the jarosites became mostly amorphous at low potentials but that Li+ may insert into empty channels at higher voltages. These results show how tuning the composition of jarosite compounds may be used to obtain different electrochemical properties, which can be used to develop improved electrode materials for Li-ion batteries.

New Hydrogen Titanium Phosphate Sulfate Electrodes for Li-ion and Na-ion Batteries
Ran Zhao, Daniel Mieritz, Dong-Kyun Seo, Candace K. Chan
J. Power Sources 2017, 343, 197-206
NASICON-type materials with general formula AxM2(PO4)3 (A = Li or Na, M = Ti, V, and Fe) are promising candidates for Li- and Na-ion batteries due to their open three-dimensional framework structure. Here we report the electrochemical properties of hydrogen titanium phosphate sulfate, H0.4Ti2(PO4)2.4(SO4)0.6 (HTPS), a new mixed polyanion material with NASICON structure. Micron-sized HTPS aggregates with crystallite grain size of ca. 23 nm are synthesized using a sol-gel synthesis in an acidic medium. The properties of the as-synthesized HTPS, ball-milled HTPS, and samples prepared as carbon composites using an in-situ glucose decomposition reaction are investigated. A capacity of 148 mAh g−1 corresponding to insertion of 2 Li+ per formula unit is observed in the ball-milled HTPS over the potential window of 1.5–3.4 V vs. Li/Li+. Lithiation at ca. 2.8 and 2.5 V is determined to occur through filling of the M1 and M2 sites, respectively. Powder X-ray diffraction (PXRD), scanning electron microscopy (SEM) and X-ray photoelectron spectroscopy (XPS) are used characterize the HTPS before and after cycling. Evaluation of the HTPS in a Na-ion cell is also performed. A discharge capacity of 93 mAh g−1 with sodiation at ca. 2.9 and 2.2 V vs. Na/Na+ is observed.

Oxidation–Reduction Assisted exfoliation of LiCoO2 into Nanosheets and Reassembly into Functional Li-ion Battery Cathodes
Qian Cheng, Ting Yang, Ying Li, Man Li, Candace K Chan
J. Mater. Chem A 2016, 4, 6902-6910
From the themed collection: Emerging Investigators 2016: Novel design strategies for new functional materials
A common approach used to obtain 2D nanosheet materials is through the exfoliation of layered compounds by osmotic, chemical/electrochemical, or mechanical means, with a proton exchange step usually implemented for materials characterized by strong interlayer ionic bonding. However, in lithium metal oxides, due to the strong adsorption of protons at Li+ sites, this approach is less effective for obtaining nanosheets with good electrochemical properties that can be used in Li-ion battery applications. Here LiCoO2 (LCO) was exfoliated into nanosheets using electrochemical oxidation followed by intercalation of tetraethylammonium cations. The nanosheets were purified using dialysis and electrophoresis. The nanosheets were successfully restacked into the O2-polytype of LCO with microwave hydrothermal assistance, indicating that non-equilibrium structures can be obtained by reassembling nanosheets. After high temperature annealing, the materials exhibited electrochemical properties characteristic of O3-type LCO with good capacity retention when passivated with atomic layer deposition Al2O3 coatings. This work shows that the proton exchange step usually required for the exfoliation of layered metal oxides can be circumvented, and moreover, that the obtained nanosheets could be restacked into functional electrode materials. This could pave the way for the synthesis of materials with novel structures and electrochemical properties, as well as facilitate the fabrication of hybrid and composite structures from different nanosheets as building blocks.

Exfoliation of LiNi1/3Mn1/3Co1/3O2 into Nanosheets Using Electrochemical Oxidation and Reassembly with Dialysis or Flocculation
Qian Cheng, Ting Yang, Man Li, Candace K. Chan
Langmuir 2017, 33, 37, 9271–9279
Two-dimensional (2D) materials such as nanosheets are increasingly attracting attention for applications in energy storage and conversion. Many conventional battery compounds have layered structures, which can facilitate the exfoliation of these materials into nanosheet morphologies. In this work, LiNi1/3Mn1/3Co1/3O2 (NMC) particles were exfoliated into nanosheets using an electrochemical oxidation method enabled by the intercalation of tetraethylammonium cations into the interlayer space. The exfoliated materials were monolayer or double-layer nanosheets with hexagonal shapes and sizes of <50 nm. Two different methods were used to reassemble the nanosheets into NMC particles: (1) a slow, dialysis-based approach and (2) direct flocculation. Characterization of the NMC materials at different stages in the exfoliation and reassembly processes was performed using compositional analysis, X-ray diffraction, electron microscopy, and electrochemical methods. The dialysis reassembly method allowed for the restacking of the nanosheets into faceted, hexagonally shaped nanoplatelets, and the flocculation approach yielded only ill-defined particles. The differences in the observed potential-dependent redox behavior and electrochemical cycling characteristics are attributed to the role of the reassembly method in the formation of phase-segregated domains, with the particles reassembled using the dialysis approach displaying the best performance.