Clathrate Electrochemistry
Overview
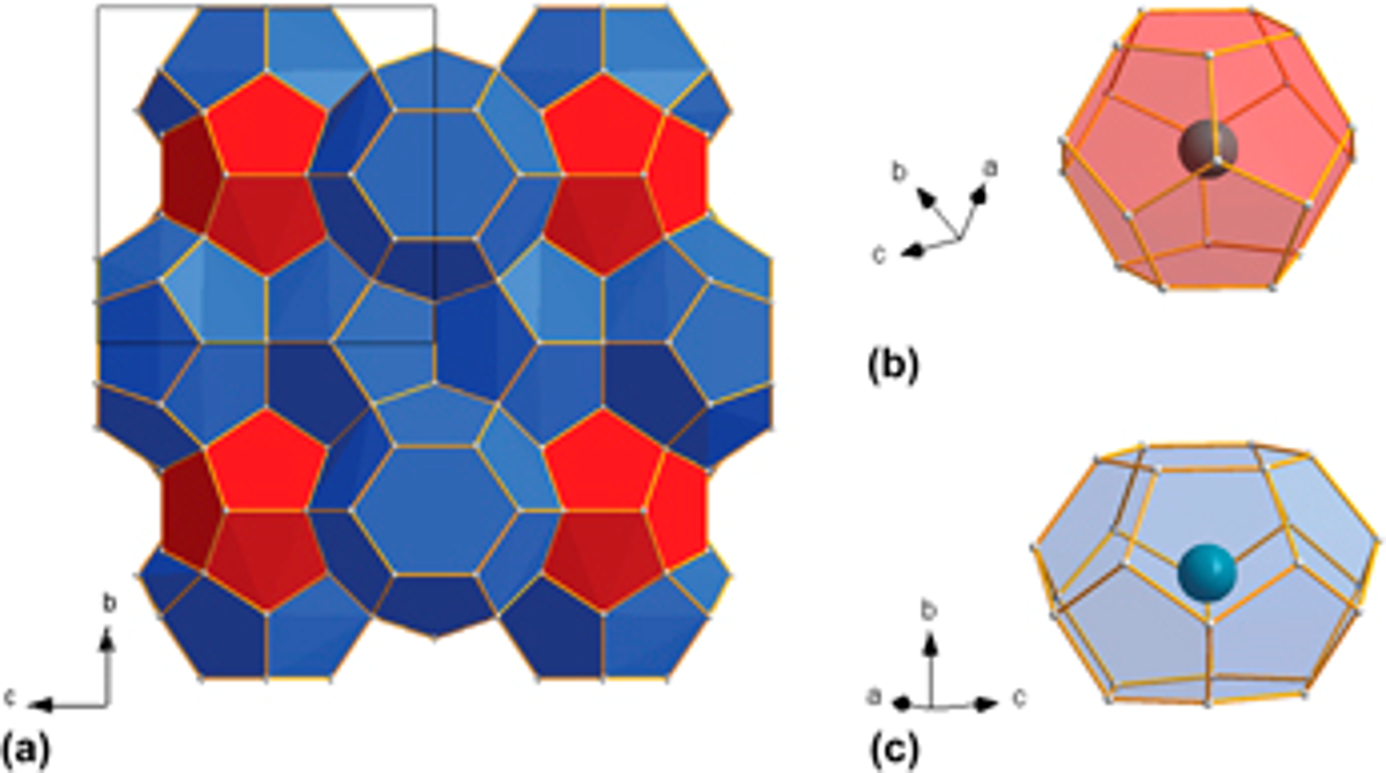
The group 14 or Tetrel (Tt) elements (Si, Ge, Sn) have received significant attention as high capacity anodes for Li-ion batteries due to their ability to alloy with large amounts of Li. Tetrel clathrates have cage-like structures composed of face-sharing Tt20, Tt24, and Tt28 polyhedra that boast strong Tt–Tt bonds with covalent sp3-character. Due to the large number of possible structures and compositions in the clathrate family, these materials have great potential to display a diverse range of electrochemical properties. With support from the National Science Foundation Solid-State Materials Chemistry program within the Division of Materials Research, we have established several important trends concerning the relationships between the clathrate structure and its electrochemical properties.
Silicon Clathrates

Electrochemical Cycling of Sodium-Filled Silicon Clathrate
Nicholas A. Wagner, Rahul Raghavan, Ran Zhao, Dr. Qun Wei, Xihong Peng, Candace K. Chan
ChemElectroChem 2014, 1, 347-353
A mixture of type I and type II silicon clathrate with sodium guest atoms is studied as a potential anode material for lithium-ion batteries. An electrochemical, structural, and first-principles analysis is conducted to understand the phase changes occurring upon lithium insertion and removal from these cage-like silicon structures.
Type I Clathrates as Novel Silicon Anodes: An Electrochemical and Structural Investigation
Ying Li, Rahul Raghavan, Nicholas A. Wagner, Stephen K. Davidowski, Loïc Baggetto, Ran Zhao, Qian Cheng, Jeffery L. Yarger, Gabriel M. Veith, Carol Ellis-Terrell, Michael A. Miller, Kwai S. Chan, Candace K. Chan
Adv. Sci. 2015, 2, 1500057
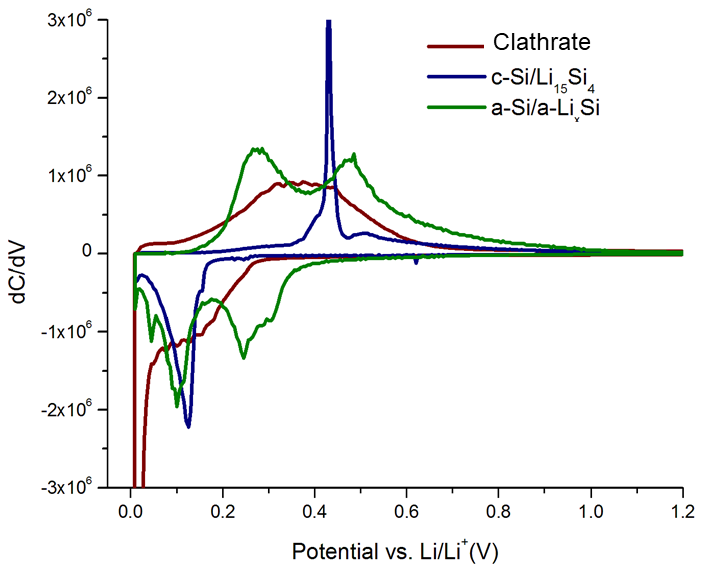
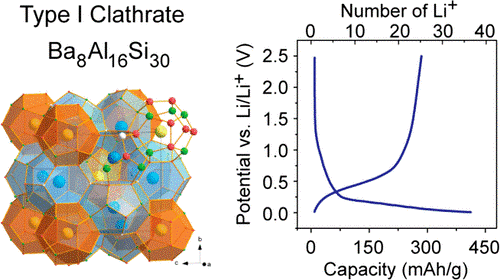
Silicon clathrates contain cage-like structures that can encapsulate various guest atoms or molecules. An electrochemical evaluation of type I silicon clathrates based on Ba8AlySi46−y as the anode material for lithium-ion batteries is presented here. Postcycling characterization with nuclear magnetic resonance and X-ray diffraction shows no discernible structural or volume changes even after electrochemical insertion of 44 Li (≈1 Li/Si) into the clathrate structure. The observed properties are in stark contrast with lithiation of other silicon anodes, which become amorphous and suffer from large volume changes. The electrochemical reactions are proposed to occur as single phase reactions at approximately 0.2 and 0.4 V versus Li/Li+ during lithiation and delithiation, respectively, distinct from diamond cubic or amorphous silicon anodes. Reversible capacities as high as 499 mAh g−1 at a 5 mA g−1 rate were observed for silicon clathrate with composition Ba8Al8.54Si37.46, corresponding to ≈1.18 Li/Si. These results show that silicon clathrates could be promising durable anodes for lithium-ion batteries.
Synthesis and Characterization of Empty Silicon Clathrates for Anode Applications in Li-ion Batteries
Kwai S. Chan, Michael A. Miller, Carol Ellis-Terrell, Candace K. Chan
MRS Advances 2016, 1, 3043-3048
Several processing methods were developed and evaluated for synthesizing empty silicon clathrates. A solution synthesis method based on the Hofmann-elimination oxidation reaction was successfully utilized to produce 20 mg of empty Si46. Half-cells using the Si46 electrodes were successfully cycled for 1000 cycles at rate of 5.3C. The capacity of the Si46 electrode in long-term tests was 675 mAh/g at the 4th cycle, but increased to 809 mAh/g at 50 cycles. The corresponding Coulombic efficiency was better than 99%. The capacity dropped from 809 to 553 mAh/g after 1000 cycles while maintaining a 99% Coulombic efficiency. In comparison, a Ba8Al8Si38 electrode could be cycled for about 200 cycles with a lower capacity and Coulombic efficiency. Potential applications of empty silicon clathrates as anode materials in Li-ion batteries are discussed.
Anodes for Lithium-Ion Batteries Based on Type I Silicon Clathrate Ba8Al16Si30 – Role of Processing on Surface Properties and Electrochemical Behavior
Ran Zhao, Svilen Bobev, Lakshmi Krishna, Ting Yang, J. Mark Weller, Hangkun Jing, Candace K. Chan
ACS Appl. Mater. Interfaces 2017, 9, 47, 41246–41257
Type I silicon clathrates based on Ba8AlySi46-y (8 < y < 12) have been studied as potential anodes for lithium-ion batteries and display electrochemical properties that are distinct from those found in conventional silicon anodes. Processing steps such as ball-milling (typically used to reduce the particle size) and acid/base treatment (used to remove nonclathrate impurities) may modify the clathrate surface structure or introduce defects, which could affect the observed electrochemical properties. In this work, we perform a systematic investigation of Ba8AlySi46-y clathrates with y ≈ 16, i.e, having a composition near Ba8Al16Si30, which perfectly satisfies the Zintl condition. The roles of ball-milling and acid/base treatment were investigated using electrochemical, X-ray diffraction, electron microscopy, X-ray photoelectron and Raman spectroscopy analysis. The results showed that acid/base treatment removed impurities from the synthesis, but also led to formation of a surface oxide layer that inhibited lithiation. Ball-milling could remove the surface oxide and result in the formation of an amorphous surface layer, with the observed charge storage capacity correlated with the thickness of this amorphous layer. According to the XRD and electrochemical analysis, all lithiation/delithiation processes are proposed to occur in single phase reactions at the surface with no discernible changes to the crystal structure in the bulk. Electrochemical impedance spectroscopy results suggest that the mechanism of lithiation is through surface-dominated, Faradaic processes. This suggests that for off-stoichiometric clathrates, as we studied in our previous work, Li+ insertion at defects or vacancies on the framework may be the origin of reversible Li cycling. However, for clathrates Ba8AlySi46-y with y ≈ 16, Li insertion in the structure is unfavorable and low capacities are observed unless amorphous surface layers are introduced by ball-milling.
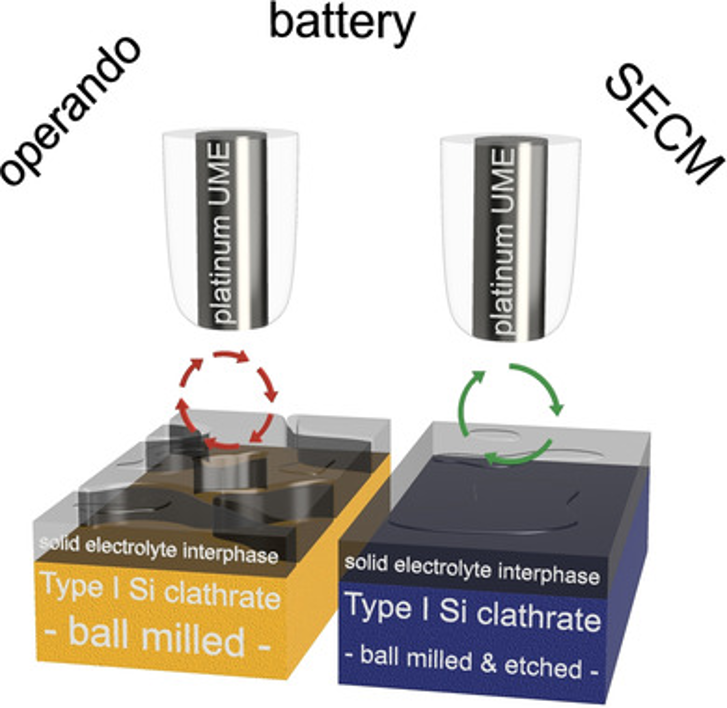
Surface Properties of Battery Materials Elucidated Using Scanning Electrochemical Microscopy: The Case of Type I Silicon Clathrate
Tsvetan Tarnev, Patrick Wilde, Andrew Dopilka,Wolfgang Schuhmann, Candace K. Chan, Edgar Ventosa
ChemElectroChem 2020, 7, 665-671
Silicon clathrates have attracted interest as potential anodes for lithium-ion batteries with unique framework structures. However, very little is known about the surface reactivity and solid electrolyte interphase (SEI) properties of clathrates. In this study, operando scanning electrochemical microscopy (SECM) is used to investigate the effect of pre-treatment on the formation dynamics and intrinsic properties of the SEI in electrodes prepared from type I Ba8Al16Si30 silicon clathrates. Although X-ray photoelectron spectroscopy (XPS) analysis does not reveal large changes in SEI composition, it is found through SECM measurements that ball-milling combined with chemical acid/base etching of the clathrates lead to a more stable and rapidly formed SEI as compared to purely ball-milled samples, resulting in enhanced Coulombic efficiency.

Electrochemical Lithium Alloying Behavior of Guest-Free Type II Silicon Clathrates
Andrew Dopilka, Amanda Childs, Svilen Bobev, Candace K. Chan
J. Phys. Chem. C 2021, 125, 35, 19110–19118
The guest-free type II Si clathrate (Si136) is an open framework polymorph of Si that displays unique electrochemical reactions with Li. Li ions are first topotactically inserted into the vacant clathrate cages, followed by an alloying reaction that forms an amorphous lithium silicide phase. The alloying reaction voltage is higher than those seen in other Si electrodes, suggesting that there are structural differences in the formed amorphous phases. Synchrotron X-ray total scattering measurements and pair distribution function analysis are employed to characterize the amorphous phases formed after lithiation. The results show that the clathrate becomes completely amorphous at an earlier stage of lithiation when compared to diamond cubic Si, forming a phase with comparatively larger amounts of Si–Si bonding. The initial insertion of Li into the clathrate cages establishes important Li diffusion paths that kinetically enable the formation of an amorphous phase with lower Li content than typically seen in other silicon-based electrodes. After the initial crystalline-to-amorphous conversion reaction, lithiation takes place via solid-solution alloying. These results demonstrate how the topotactic insertion of Li into an alloying host can kinetically enable modified reaction pathways leading to more homogeneous lithiation throughout the electrode, which is beneficial for Li-ion battery applications.
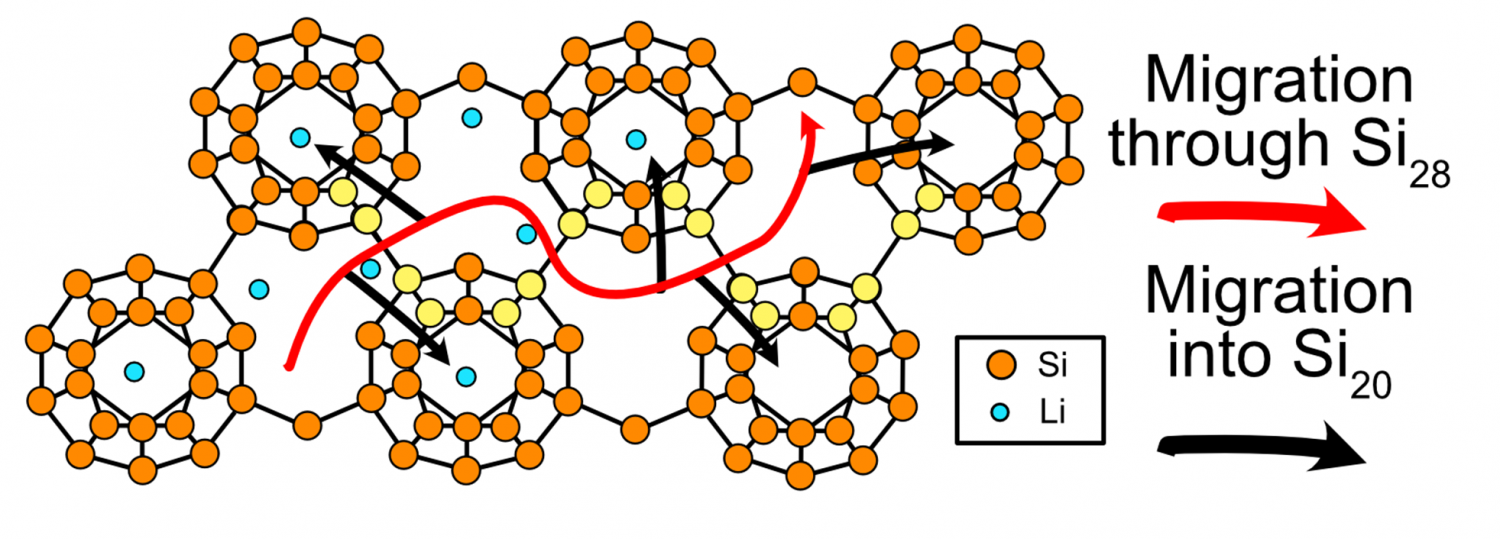
Structural Origin of Reversible Li Insertion in Guest‐Free, Type‐II Silicon Clathrates
Andrew Dopilka, J. Mark Weller, Alexander Ovchinnikov, Amanda Childs, Svilen Bobev, Xihong Peng, Candace K Chan
Adv. Energy Sustainability Res. 2021, 2, 2000114
The guest-free, type-II Si clathrate (Si136) is an open cage polymorph of Si with structural features amenable to electrochemical Li storage. However, the detailed mechanism for reversible Li insertion and migration within the vacant cages of Si136 is not established. Herein, X-ray characterization and density functional theory (DFT) calculations are used to understand the structural origin of electrochemical Li insertion into the type-II clathrate structure. At low Li content, instead of alloying with Si, topotactic Li insertion into the empty cages occurs at ≈0.3 V versus Li/Li+ with a capacity of ≈231 mAh g−1 (corresponding to composition Li32Si136). A synchrotron powder X-ray diffraction analysis of electrodes after lithiation shows evidence of Li occupation within the Si20 and Si28 cages and a volume expansion of 0.22%, which is corroborated by DFT calculations. Nudged elastic band calculations suggest a low barrier (0.2 eV) for Li migration through interconnected Si28 cages, whereas there is a higher barrier for Li migration into Si20 cages (2.0 eV). However, if Li is present in a neighboring cage, a cooperative migration pathway with a barrier of 0.65 eV is possible. The results show that the type-II Si clathrate displays unique electrochemical properties for potential applications as Li-ion battery anodes.
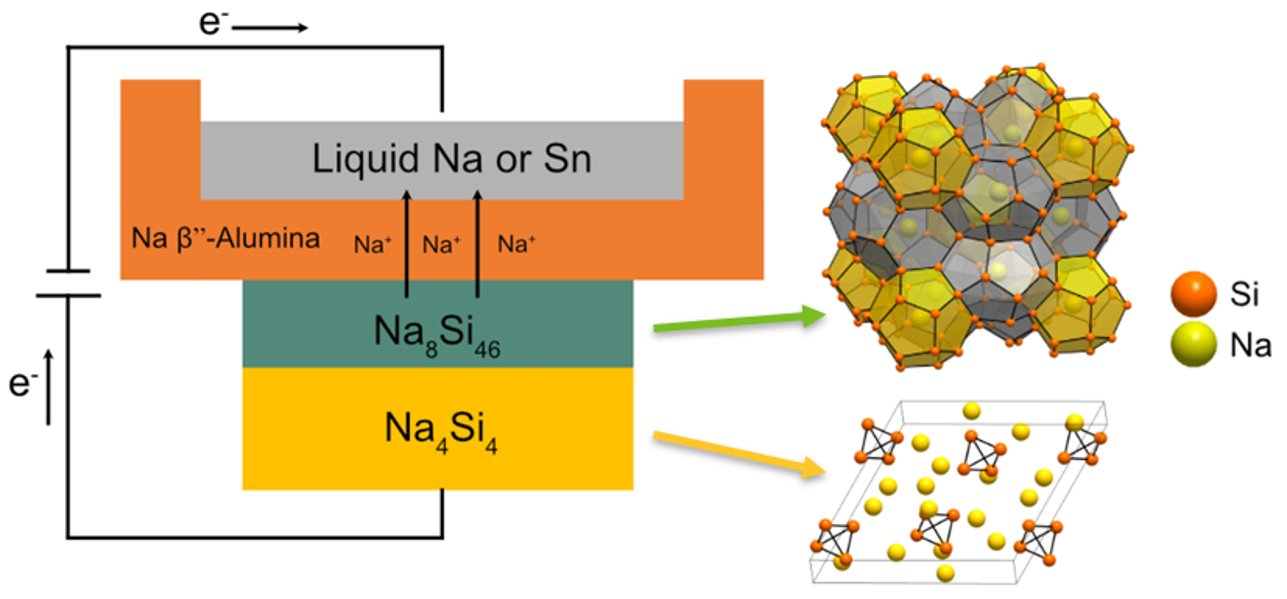
Solid-State Electrochemical Synthesis of Silicon Clathrates Using a Sodium-Sulfur Battery Inspired Approach
Andrew Dopilka, Amanda Childs, Svilen Bobev, Candace K Chan
J. Electrochem. Soc. 2021, 168, 020516
Focus Issue on Future of Intercalation Chemistry for Energy Storage and Conversion in Honor of M. Stanley Whittingham
Clathrates of Tetrel elements (Si, Ge, Sn) have attracted interest for their potential use in batteries and other applications. Sodium-filled silicon clathrates are conventionally synthesized through thermal decomposition of the Zintl precursor Na4Si4, but phase selectivity of the product is often difficult to achieve. Herein, we report the selective formation of the type I clathrate Na8Si46 using electrochemical oxidation at 450 °C and 550 °C. A two-electrode cell design inspired by high-temperature sodium-sulfur batteries is employed, using Na4Si4 as working electrode, Na β”-alumina solid electrolyte, and counter electrode consisting of molten Na or Sn. Galvanostatic intermittent titration is implemented to observe the oxidation characteristics and reveals a relatively constant cell potential under quasi-equilibrium conditions, indicating a two-phase reaction between Na4Si4 and Na8Si46. We further demonstrate that the product selection and morphology can be altered by tuning the reaction temperature and Na vapor pressure. Room temperature lithiation of the synthesized Na8Si46 is evaluated for the first time, showing similar electrochemical characteristics to those in the type II clathrate Na24Si136. The results show that solid-state electrochemical oxidation of Zintl phases at high temperatures can lead to opportunities for more controlled crystal growth and a deeper understanding of the formation processes of intermetallic clathrates.
Germanium Clathrates
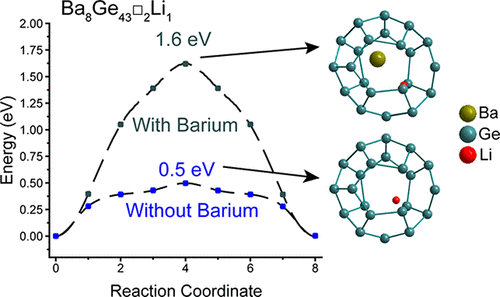
Experimental and Computational Study of the Lithiation of Ba8AlyGe46-y Based Type I Germanium Clathrates
Andrew Dopilka, Ran Zhao, J. Mark Weller, Svilen Bobev, Xihong Peng, Candace K. Chan
ACS Appl. Mater. Interfaces 2018, 10, 44, 37981–37993
In this work, we investigate the electrochemical properties of Ba8AlyGe46–y (y = 0, 4, 8, 12, 16) clathrates prepared by arc-melting. These materials have cage-like structures with large cavity volumes and can also have vacancies on the Ge framework sites, features which may be used to accommodate Li. Herein, a structural, electrochemical, and theoretical investigation is performed to explore these materials as anodes in Li-ion batteries, including analysis of the effect of the Al content and framework vacancies on the observed electrochemical properties. Single-crystal X-ray diffraction (XRD) studies indicate the presence of vacancies at the 6c site of the clathrate framework as the Al content decreases, and the lithiation potentials and capacities are observed to decrease as the degree of Al substitution increases. From XRD, electrochemical, and transmission electron microscopy analysis, we find that all of the clathrate compositions undergo two-phase reactions to form Li-rich amorphous phases. This is different from the behavior observed in Si clathrate analogues, where there is no amorphous phase transition during electrochemical lithiation nor discernible changes to the lattice constant of the bulk structure. From density functional theory calculations, we find that Li insertion into the three framework vacancies in Ba8Ge43 is energetically favorable, with a calculated lithiation voltage of 0.77 V versus Li/Li+. However, the calculated energy barrier for Li diffusion between vacancies and around Ba guest atoms is at least 1.6 eV, which is too high for significant room-temperature diffusion. These results show that framework vacancies in the Ge clathrate structure are unlikely to significantly contribute to lithiation processes unless the Ba guest atoms are absent, but suggest that guest atom vacancies could open diffusion paths for Li, allowing for empty framework positions to be occupied.

Understanding the Amorphous Lithiation Pathway of the Type I Ba8Ge43 Clathrate with Synchrotron X-ray Characterization
Andrew Dopilka, Amanda Childs, Svilen Bobev, Candace K. Chan
Chem. Mater. 2020, 32, 21, 9444–9457
Tetrel (Tt = Si, Ge, and Sn) clathrates have highly tunable host–guest structures and have been investigated as novel electrode materials for Li-ion batteries. However, there is little understanding of how the clathrate structure affects the lithiation processes and phase evolution. Herein, the electrochemical lithiation pathway of type I clathrate Ba8Ge43 is investigated with synchrotron X-ray diffraction (XRD) and pair distribution function (PDF) analyses and compared to the lithiation of germanium with a diamond cubic structure (α-Ge). The results confirm previous laboratory XRD studies showing that Ba8Ge43 goes through a solely amorphous phase transformation, which contrasts with the crystalline phase transformations that take place during lithiation of micrometer-sized α-Ge particles. The local structure of framework-substituted clathrate Ba8Al16Ge30 after lithiation is found to proceed through an amorphous phase transformation similar to that in Ba8Ge43. In situ PDF and XRD during heating show that the amorphous phases derived from lithiation of Ba8Ge43 are structurally related to various Li–Ge phases and crystallize at low temperatures (350–420 K). We conclude that the Ba atoms inside the clathrate structure act to break up the long-range ordering of Li–Ge clusters and kinetically prevent the nucleation and growth of bulk crystalline phases. The amorphous phase evolution of the clathrate structure during lithiation results in electrochemical properties distinct from those in α-Ge, such as a single-phase reaction mechanism and lower voltage, suggesting possible advantages of clathrates over elemental phases for use as anodes in Li-ion batteries.

Synthesis of Type II Ge and Ge–Si Alloyed Clathrates Using Solid-State Electrochemical Oxidation of Zintl Phase Precursors
Andrew Dopilka, Alexander Ovchinnikov, Amanda Childs, Svilen Bobev, Xihong Peng, Candace K. Chan
Inorg. Chem. 2022, 61, 31, 12363–12372
Germanium clathrates with the type II structure are open-framework materials that show promise for various applications, but the difficulty of achieving phase-pure products via traditional synthesis routes has hindered their development. Herein, we demonstrate the synthesis of type II Ge clathrates in a two-electrode electrochemical cell using Na4Ge4–ySiy (y = 0, 1) Zintl phase precursors as the working electrode, Na metal as the counter/reference electrode, and Na-ion conducting β″-alumina as the solid electrolyte. The galvanostatic oxidation of Na4Ge4 resulted in voltage plateaus around 0.34–0.40 V vs Na/Na+ with the formation of different products depending on the reaction temperature. When using Na4Ge3Si as a precursor, nearly phase-pure, alloyed type II Ge–Si clathrate was obtained at 350 °C. The Na atoms in the large (Ge,Si)28 cages of the clathrate occupied off-centered positions according to Rietveld refinement and density functional theory calculations. The results indicate that electrochemical oxidation of Zintl phase precursors is a promising pathway for synthesizing Ge clathrates with type II structure and that Si alloying of the Zintl phase precursor can promote selective clathrate product formation over other phases.
Tin Clathrates

Structural and Electrochemical Properties of Type VIII Ba8Ga16−δSn30+δ Clathrate (δ ≈ 1) during Lithiation
Andrew Dopilka, Amanda Childs, Alexander Ovchinnikov, Ran Zhao, Svilen Bobev, Xihong Peng, Candace K. Chan
ACS Appl. Mater. Interfaces 2021, 13, 36, 42564–42578
Clathrates of the tetrel (Tt = Si, Ge, Sn) elements are host–guest structures that can undergo Li alloying reactions with high capacities. However, little is known about how the cage structure affects the phase transformations that take place during lithiation. To further this understanding, the structural changes of the type VIII clathrate Ba8Ga16−δSn30+δ (δ ≈ 1) during lithiation are investigated and compared to those in β-Sn with ex situ X-ray total scattering measurements and pair distribution function (PDF) analysis. The results show that the type VIII clathrate undergoes an alloying reaction to form Li-rich amorphous phases (LixBa0.17Ga0.33Sn0.67, x = 2–3) with local structures similar to those in the crystalline binary Li–Sn phases that form during the lithiation of β-Sn. As a result of the amorphous phase transition, the type VIII clathrate reacts at a lower voltage (0.25 V vs Li/Li+) compared to β-Sn (0.45 V) and goes through a solid-solution reaction after the initial conversion of the crystalline clathrate phase. Cycling experiments suggest that the amorphous phase persists after the first lithiation and results in considerably better cycling than in β-Sn. Density functional theory (DFT) calculations suggest that topotactic Li insertion into the clathrate lattice is not favorable due to the high energy of the Li sites, which is consistent with the experimentally observed amorphous phase transformation. The local structure in the clathrate featuring Ba atoms surrounded by a cage of Ga and Sn atoms is hypothesized to kinetically circumvent the formation of Li–Sn or Li–Ga crystalline phases, which results in better cycling and a lower reaction voltage. Based on the improved electrochemical performance, clathrates could act as tunable precursors to form amorphous Li alloying phases with novel electrochemical properties.
Ab Initio Studies

First-Principles Study of Lithiation of Type I Ba-doped Silicon Clathrates
Xihong Peng, Qun Wei, Ying Li, Candace K. Chan
J. Phys. Chem. C 2015, 119, 51, 28247–28257
Silicon clathrate materials, previously known for their superconducting and thermoelectric characteristics, have also recently been investigated for their electrochemical properties as anodes for lithium-ion batteries due to their unique cage structure and ability to incorporate extrinsic guest atoms. To better understand the preferred structures for small degrees of lithiation, first-principles density functional theory (DFT) was used to investigate the type I clathrate compounds Si46, LixBaySi46 (0 ≤ x ≤ 8; y = 6, 8), and LixBayAl6Si40 (0 ≤ x ≤ 8; y = 6). The formation energies, electronic band structures, and density of states (DOS) were calculated. Lithium occupation in framework vacancies, empty and Ba-occupied cage cavities, and near the pentagonal and hexagonal faces of the clathrate polyhedra was considered. The data showed that Li insertion into framework or Ba vacancies could stabilize the clathrate structure. Silicon substitution by Al lowered the formation energies of the lithiated compounds and mitigated the calculated volume increase upon lithiation. The results also showed that it is energetically feasible for multiple guest atoms to be placed in the Si24 cages. Changes in the clathrate atomic structure (e.g., bond lengths and angles) and electronic structure were highly dependent on the location of the Li and guest atom spacing within the clathrate framework. The results from this study can elucidate the preferred structural configurations for Li in type I, Ba-doped silicon clathrates and also be informative for efforts related to understanding the structures obtained after electrochemical insertion of lithium into silicon clathrates.
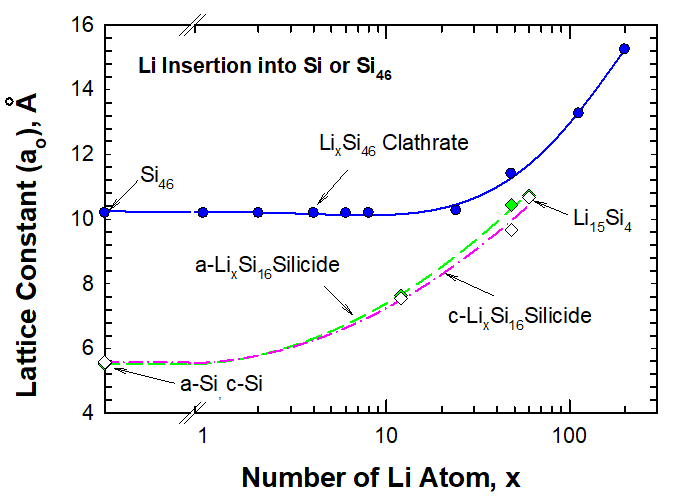
First Principles and Experimental Studies of Empty Si46 as Anode Materials for Li-ion Batteries
Kwai S. Chan, Michael A. Miller, Wuwei Liang, Carol Ellis-Terrell, Candace K. Chan
J. Mater. Res. 2016, 31, 3657-3665
The objective of this investigation was to utilize the first-principles molecular dynamics computational approach to investigate the lithiation characteristics of empty silicon clathrates (Si46) for applications as potential anode materials in lithium-ion batteries. The energy of formation, volume expansion, and theoretical capacity were computed for empty silicon clathrates as a function of Li. The theoretical results were compared against experimental data of long-term cyclic tests performed on half-cells using electrodes fabricated from Si46 prepared using a Hofmann-type elimination–oxidation reaction. The comparison revealed that the theoretically predicted capacity (of 791.6 mAh/g) agreed with experimental data (809 mAh/g) that occurred after insertion of 48 Li atoms. The calculations showed that overlithiation beyond 66 Li atoms can cause large volume expansion with a volume strain as high as 120%, which may correlate to experimental observations of decreasing capacities from the maximum at 1030 mAh/g to 553 mA h/g during long-term cycling tests. The finding suggests that overlithiation beyond 66 Li atoms may have caused damage to the cage structure and led to lower reversible capacities.

Ab Initio Investigation of Li and Na Migration in Guest Free, Type I Clathrates
Andrew Dopilka, Xihong Peng, Candace K. Chan
J. Phys. Chem. C 2019, 123, 37, 22812–22822
Guest-free, type I clathrates with formula Tt46 (Tt = Si, Ge, Sn) are comprised of open, cage-like frameworks with the potential for facile Li or Na conduction. Herein, ab initio density functional theory (DFT) is used to evaluate the ionic mobility of Li and Na through the clathrate crystal structures. The favorable Li and Na positions inside the clathrate structures are determined, and the migration pathways and barriers are evaluated using the nudged elastic band (NEB) method. The results show that it is energetically favorable for a Li atom to occupy the center position inside the small Tt20 cages while preferring the off-center positions in the larger Tt24 cages. The lowest Li migration barriers are found to be 0.35, 0.13 and 0.37 eV for Si46, Ge46, and Sn46, respectively, with the dominant diffusion pathway along channels of Tt24 cages connected by hexagonal faces. Li accessibility to the Si20 cage in Si46 appears to be restricted in the dilute regime due to a high energy barrier (2.0 eV) except for the case in which Li atoms are present in adjacent cages; this lowers the migration barrier to 0.77 eV via a mechanism where a Si–Si bond is temporarily broken. In contrast, Na atoms show preference for the cage centers and display higher migration barriers than Li. Overall, the Tt24 channel sizes in the guest-free, type I clathrates are ideal for fast Li diffusion, while Na is too large to migrate effectively between cages. The energy landscape for Li inside the type I clathrates is uniquely different than that in diamond cubic structures, leading to significantly lower energy barriers for Li migration. These results suggest that open frameworks of intermetallic elements may enable facile Li migration and have potential use as Li-ion battery anodes.