Garnet Research
Overview
Lithium-ion batteries are ubiquitous in laptops and cell phones and will gain more use in transportation applications in the near future. However, these batteries suffer from safety issues originating from the flammable liquid electrolyte that is used to transport lithium ions within the battery. Solid electrolytes can replace flammable liquid electrolytes, display improved stability, and provide a physical barrier against short circuits caused by lithium dendrite propagation, leading to improved reliability, prolonged battery life, and increased energy density. Lithium lanthanum zirconate (Li7La3Zr2O12,LLZO) is a lithium conducting ceramic with many promising characteristics for potential use as solid electrolyte in lithium metal batteries. This research supported by the National Science Foundation CAREER award (DMR-1553519) was motivated by the desire to obtain better fundamental understanding of structure-property relationships in lithium-ion conducting ceramics, particularly in LLZO nanostructured materials. This project investigated novel LLZO nanostructures and composites with unique nanoscale properties that can improve their ion conductivity and integration into safer, all-solid-state batteries. The major goals of this research project were to correlate materials properties such as the composition, grain boundary structure, and crystal phase with the sintering properties and ionic conductivity of LLZO.
Electrospun Solid Electrolyte Nanowires
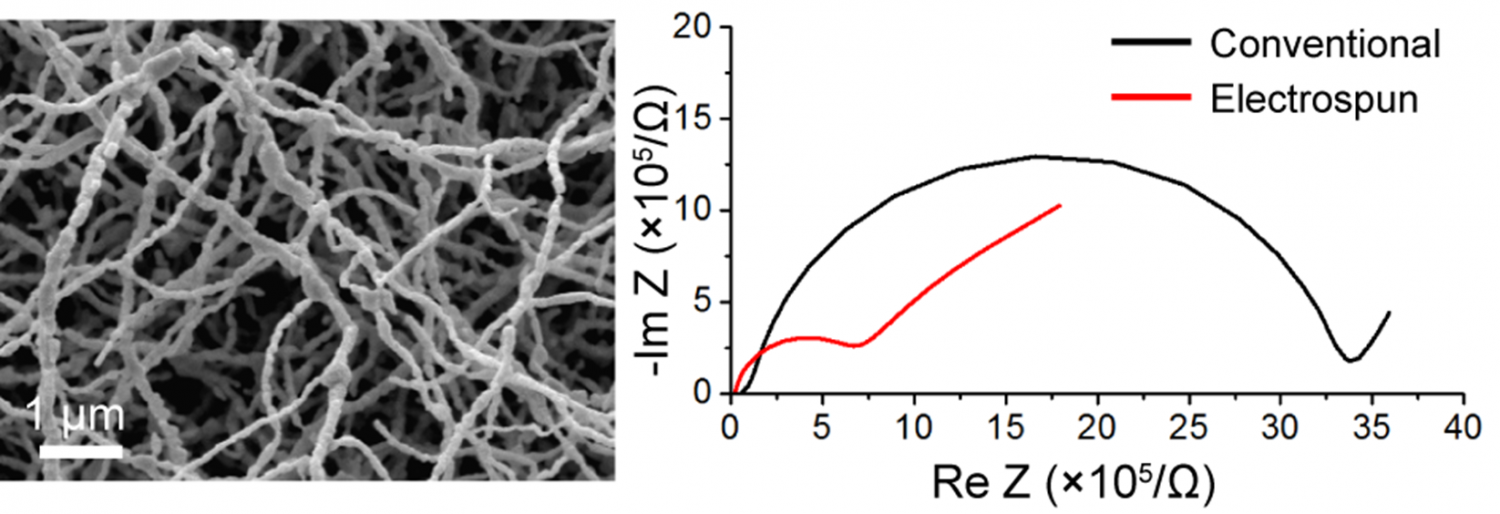
Enhanced Lithium Ion Conductivity in Lithium Lanthanum Titanate Solid Electrolyte Nanowires Prepared by Electrospinning
Ting Yang, Ying Li, Candace K. Chan
J. Power Sources 2015, 287, 164-169
Solid electrolytes have great potential to address the safety issues of Li-ion batteries, but better synthesis methods are still required for ceramics such as lithium lanthanum titanate (LLTO) since current techniques require high-temperature calcination for long times. Here we report a new approach that utilizes electrospinning to prepare phase-pure polycrystalline LLTO nanowires with well-crystallized tetragonal structure after only 3 h calcination at 1000 °C. Pellets prepared from the electrospun LLTO nanowires had higher density, less void space, and higher Li+ conductivity compared to those comprised of LLTO prepared with conventional sol–gel methods. This work demonstrates the potential that electrospinning can provide towards improving the properties of sol–gel derived ceramics.
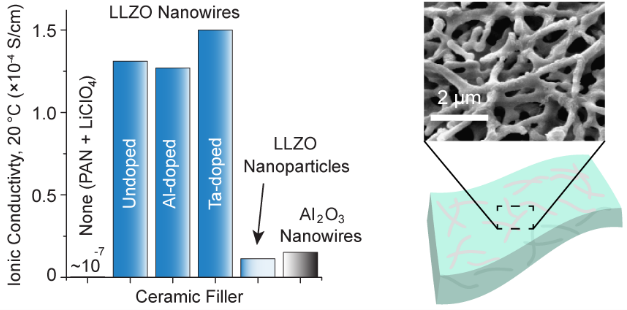
Composite Polymer Electrolytes with Li7La3Zr2O12 Garnet-Type Nanowires as Ceramic Fillers: Mechanism of Conductivity Enhancement and Role of Doping and Morphology
Ting Yang, Jin Zheng, Qian Cheng, Yan-Yan Hu, Candace K. Chan
ACS Appl. Mater. Interfaces 2017, 9, 26, 21773–21780
Composite polymer solid electrolytes (CPEs) containing ceramic fillers embedded inside a polymer-salt matrix show great improvements in Li+ ionic conductivity compared to the polymer electrolyte alone. Lithium lanthanum zirconate (Li7La3Zr2O12, LLZO) with a garnet-type crystal structure is a promising solid Li+ conductor. We show that by incorporating only 5 wt % of the ceramic filler comprising undoped, cubic-phase LLZO nanowires prepared by electrospinning, the room temperature ionic conductivity of a polyacrylonitrile-LiClO4-based composite is increased 3 orders of magnitude to 1.31 × 10–4 S/cm. Al-doped and Ta-doped LLZO nanowires are also synthesized and utilized as fillers, but the conductivity enhancement is similar as for the undoped LLZO nanowires. Solid-state nuclear magnetic resonance (NMR) studies show that LLZO NWs partially modify the PAN polymer matrix and create preferential pathways for Li+ conduction through the modified polymer regions. CPEs with LLZO nanoparticles and Al2O3 nanowire fillers are also studied to elucidate the role of filler type (active vs passive), LLZO composition (undoped vs doped), and morphology (nanowire vs nanoparticle) on the CPE conductivity. It is demonstrated that both intrinsic Li+ conductivity and nanowire morphology are needed for optimal performance when using 5 wt % of the ceramic filler in the CPE.
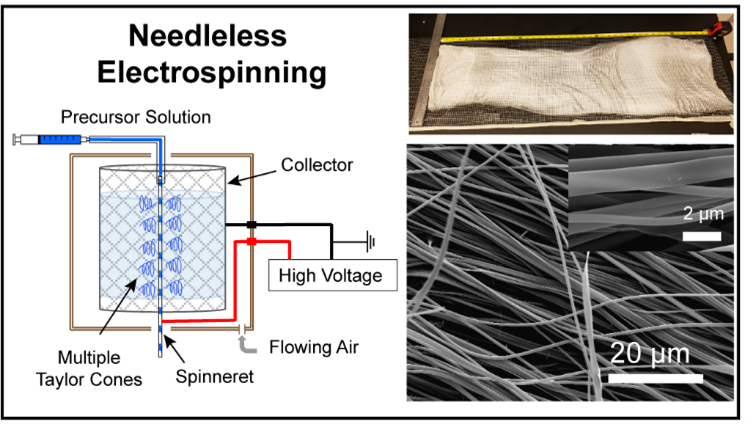
Needleless Electrospinning for High Throughput Production of Li7La3Zr2O12 Solid Electrolyte Nanofibers
Tanner Rosenthal, J. Mark Weller, Candace K. Chan
Ind. Eng. Chem. Res. 2019, 58, 37, 17399–17405
Li7La3Zr2O12 (LLZO) is a promising ceramic Li-ion conductor that has been successfully prepared in nanowire morphology using electrospinning from polymer/sol–gel solutions followed by calcination. However, conventional single-needle electrospinning has low production rates <0.1 g/h, making scale-up of the materials for applications in solid-state electrolytes challenging. Herein, needleless electrospinning using a twisted wire spinneret is employed to prepare Al-doped LLZO (ALLZO). We find that the appropriate precursor solutions for needleless electrospinning require much lower viscosity and are more sensitive to environmental conditions than those used in single-needle electrospinning. The as-formed nanofibers display a nanoribbon morphology, and calcination at 700 °C for 2 h in air results in phase pure ALLZO interconnected nanostructures with a cubic crystal structure. The results show that needleless electrospinning is an effective approach for preparing as-spun nanofibers with yields of ∼1 g/h possible, providing a higher throughput route toward Li+ conducting nanostructures for solid-state battery applications.
Molten Salt Synthesis
Synthesis of Fine Cubic Li7La3Zr2O12 Powders in Molten LiCl–KCl Eutectic and Facile Densification by Reversal of Li+/H+ Exchange
J. Mark Weller, Justin A. Whetten, Candace K. Chan
ACS Appl. Energy Mater. 2018, 1, 552-560
Recently, solid-state electrolytes have been a highly active area of research for future Li-ion batteries due to the potential for drastically improved energy density and safety. Among these materials, garnet structured lithium lanthanum zirconate (Li7La3Zr2O12, LLZO) shows particular promise owing to the high ionic conductivity of its cubic polymorph, inertness, and electrochemical stability against metallic lithium. Herein we report the facile preparation of phase-pure, cubic LLZO via molten salt synthesis in a eutectic mixture of LiCl–KCl at 900 °C. Fine powders of Al- and Ga-doped LLZO were obtained with primary particle sizes ranging from 0.3 to 3 μm. Depending on the consolidation conditions, pellets with up to 86% relative density could be obtained, with Li+ conductivity values ranging from 0.230 to 0.371 mS cm–1. It is also observed that while the effect of hydration has a profoundly deleterious effect on sintering and densification, this effect can be mitigated by the simple addition of LiOH before sintering to reverse hydration and aid densification. Qualitative discussions on the mechanisms of LLZO formation in the molten salt medium are discussed, in addition to implications for scalable processing of LLZO electrolytes.
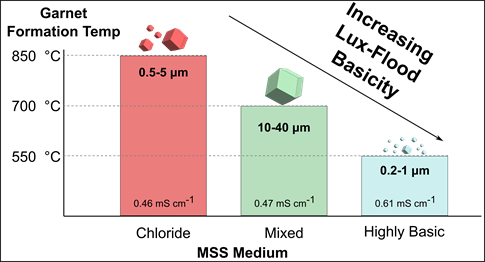
Reduction in Formation Temperature of Ta-Doped Lithium Lanthanum Zirconate by Application of Lux–Flood Basic Molten Salt Synthesis
J. Mark Weller and Candace K. Chan
ACS Appl. Energy Mater. 2020, 3, 6466-6475
Garnets such as Li7La3Zr2O12 (LLZO) are important Li+ conducting ceramics for potential use as solid electrolytes in solid-state batteries. However, LLZO is predominately prepared by using solid-state reaction methods, despite the high energy cost, multiple steps involved, and large particle sizes of the resultant material. Herein, molten salt synthesis (MSS) is applied to prepare Ta-doped LLZO (Li6.4La3Zr1.4Ta0.6O12, LLZTO), demonstrating that control over the Lux–Flood basicity of the molten salt medium enables drastic reduction in the formation temperature relative to other synthetic methods. Each of the reaction media investigated, including eutectic LiCl–KCl, a mixture of LiCl–LiOH, and highly basic ternary mixtures of LiNO3–LiOH–Li2O2, can be used to synthesize LLZTO under the appropriate experimental conditions. In the last case, garnet powders with predominately submicrometer particle sizes are obtained at temperatures as low as 550 °C. Sintered LLZTO pellets with high room temperature ionic conductivity can be obtained by using powders from each MSS method. LLZTO powders synthesized from the highly basic melts show good densification due to the small particle sizes (0.2–1 μm) and exhibit total ionic conductivity as high as 0.61 mS cm–1. The results show that molten salt synthesis in media with high Lux–Flood basicity is an attractive low-temperature synthetic approach to achieving highly conducting garnet electrolytes.

Highly Conductive Garnet-Type Electrolytes: Access to Li6.5La3Zr1.5Ta0.5O12 Prepared by Molten Salt and Solid-State Methods
Pavan Badami, J. Mark Weller, Abdul Wahan, Günther Redhammer, Lukas Ladenstein, Daniel Rettenwander, Martin Wilkening, Candace K. Chan, Arunachala Nadar Mada Kannan
ACS Appl. Mater. Interfaces 2020, 12, 43, 48580–48590
Tantalum-doped garnet (Li6.5La3Zr1.5Ta0.5O12, LLZTO) is a promising candidate to act as a solid electrolyte in all-solid-state batteries owing to both its high Li+ conductivity and its relatively high robustness against the Li metal. Synthesizing LLZTO using conventional solid-state reaction (SSR) requires, however, high calcination temperature (>1000 °C) and long milling steps, thereby increasing the processing time. Here, we report on a facile synthesis route to prepare LLZTO using a molten salt method (MSS) at lower reaction temperatures and shorter durations (900 °C, 5 h). Additionally, a thorough analysis on the properties, i.e., morphology, phase purity, and particle size distribution of the LLZTO powders, is presented. LLZTO pellets, either prepared by the MSS or the SSR method, that were sintered in a Pt crucible showed Li+ ion conductivities of up to 0.6 and 0.5 mS cm–1, respectively. The corresponding activation energy values are 0.37 and 0.38 eV, respectively. The relative densities of the samples reached values of approximately 96%. For comparison, LLZTO pellets sintered in alumina crucibles or with γ-Al2O3 as sintering aid revealed lower ionic conductivities and relative densities with abnormal grain growth. We attribute these observations to the formation of Al-rich phases near the grain boundary regions and to a lower Li content in the final garnet phase. The MSS method seems to be a highly attractive and an alternative synthetic approach to SSR route for the preparation of highly conducting LLZTO-type ceramics.
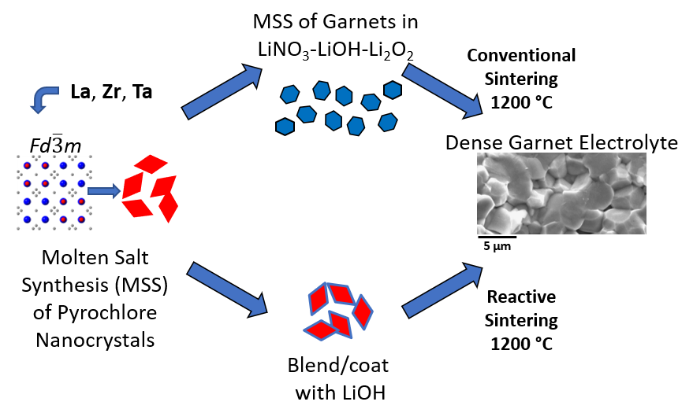
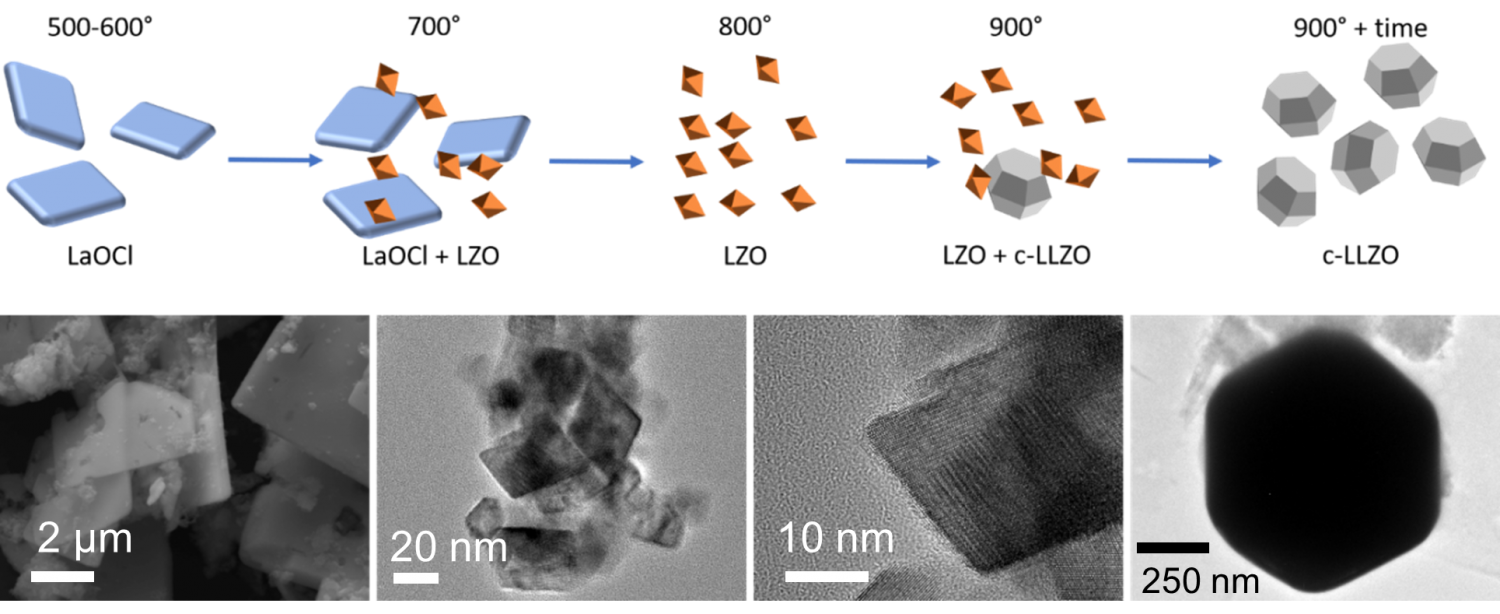
Pyrochlore Nanocrystals as Versatile Quasi-Single-Source Precursors to Lithium Conducting Garnets
J. Mark Weller and Candace K. Chan
J. Mater. Chem. A 2020, 8, 17405-17410

Observation of Elemental Inhomogeneity and Its Impact on Ionic Conductivity in Li-Conducting Garnets Prepared with Different Synthesis Methods
J Mark Weller, Andrew Dopilka, Candace K. Chan
Adv. Energy Sustainability Res. 2021, 2, 2000109
Tantalum-doped lithium lanthanum zirconate garnet (Li7−xLa3Zr2−xTaxO12 [LLZTO]) has received interest as a solid electrolyte for solid-state lithium batteries due to its good electrochemical properties and ionic conductivity. However, the source of discrepancies for reported values of ionic conductivity in nominally or nearly equivalent compositions of LLZTO is not completely clear. Herein, synthesis-related factors that may contribute to the differences in performance of garnet electrolytes are systematically characterized. The conductivity of samples with composition Li6.4La3Zr1.4Ta0.6O12 prepared by various methods including solid-state reaction (SSR), combustion, and molten salt synthesis is compared. Varying levels of elemental inhomogeneity, comprising a variation in Ta and Zr content on the level of individual LLZTO particles, are identified. The elemental inhomogeneity is found to be largely preserved even after high-temperature sintering and correlated with reduced ionic conductivity. It is shown that the various synthesis and processing-related variables in each of the preparation methods play a role in these compositional variations, and that even LLZTO synthesized via conventional, high-temperature SSR can exhibit substantial variability in local composition. However, by improving reagent mixing and using LLZTO powder with low agglomeration and small particle size distribution, the compositional uniformity, and hence, ionic conductivity, of sintered garnet electrolytes can be improved.
Sol-Gel Methods
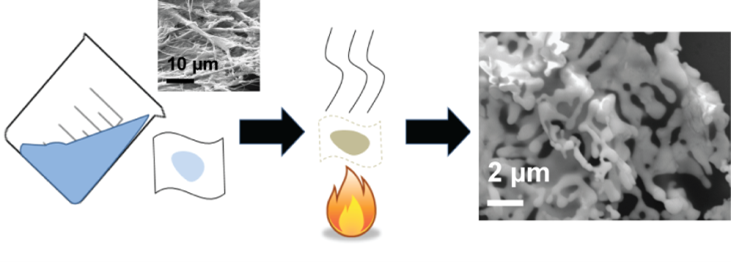
Preparation of Nano- and Microstructured Garnet Li7La3Zr2O12 Solid Electrolytes for Li-Ion Batteries via Cellulose Templating
Zachary D. Gordon, Ting Yang, Guilherme Bruno Gomes Morgado, Candace K. Chan
ACS Sustainable Chem. Eng. 2016, 4, 12, 6391–6398
Lithium lanthanum zirconate (LLZO) is a promising Li+ ion conductor for applications as a ceramic solid electrolyte in all-solid-state lithium batteries. However, the tetragonal and cubic phases of LLZO differ in lithium ionic conductivity by several orders of magnitude with extrinsic dopants or nanostructuring often required to stabilize the high conductivity cubic phase at room temperature. Here, we show that nanostructured LLZO can be prepared by templating onto various cellulosic fibers, including laboratory Kimwipes, Whatman filter paper, and nanocellulose fibrils, followed by calcination at 700–800 °C. The effect of templating material, calcination temperature, calcination time, and heating ramp rate on the LLZO crystal structure and morphology were thoroughly investigated. Templating was determined to be an effective method for controlling the LLZO size and morphology, and low calcination times and ramp rates were found to favor the formation of small ligaments. Furthermore, it was verified that cubic phase stabilization occurred for LLZO with ligaments of size less than 1 μm on average without the use of extrinsic dopants. This work provides more information regarding the size dependence of cubic LLZO stabilization that was not previously investigated in detail, and cellulosic templating is shown to be a viable route toward the scalable, sustainable synthesis of LLZO solid electrolytes.
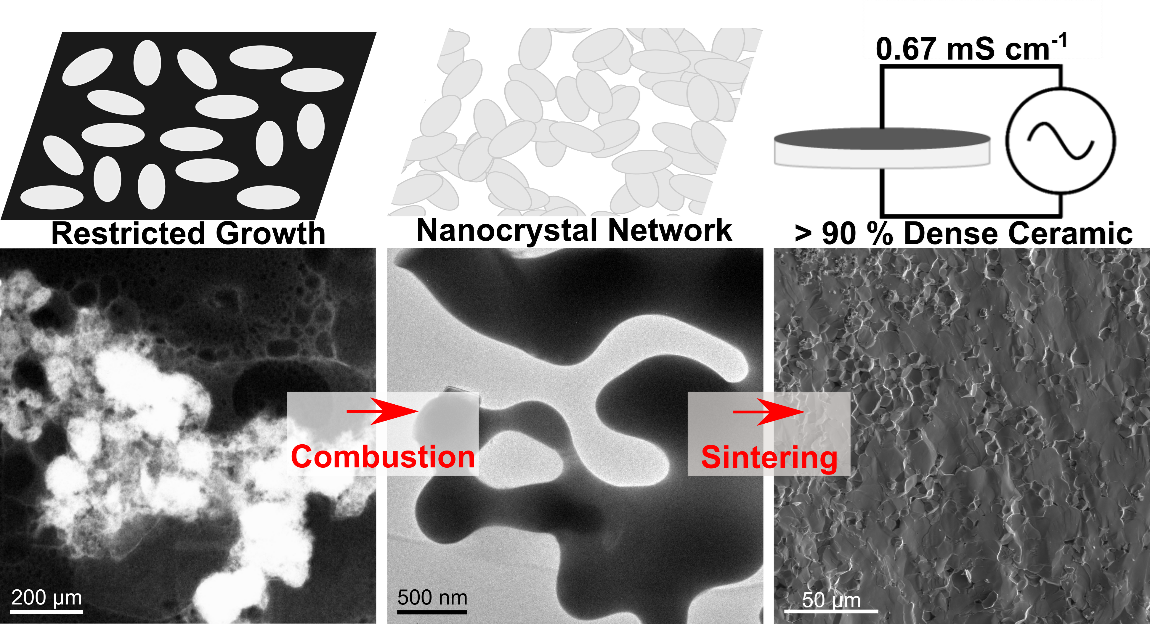
Nonaqueous Polymer Combustion Synthesis of Cubic Li7La3Zr2O12 Nanopowders
J. Mark Weller, Justin A. Whetten, and Candace K. Chan
ACS Appl. Mater. Interfaces 2020,12, 953-962
Garnet-type lithium lanthanum zirconate (Li7La3Zr2O12, LLZO) shows great promise as a solid electrolyte for future solid-state lithium batteries as it possesses a uniquely beneficial combination of high ionic conductivity, electrochemical stability against metallic lithium, and generally low reactivity in ambient conditions. Conventionally synthesized by using solid-state reactions, LLZO powders have also been prepared by using variations of sol–gel or combustion synthesis with sacrificial organic templates or polymers containing metal nitrate precursors. Herein, a novel nonaqueous polymer (NAP) method using metalorganic precursors and poly(vinylpyrrolidone) is demonstrated to easily form LLZO nanopowders. Compared to similar techniques using aqueous solutions with metal nitrates, the NAP method confers greater control over synthesis conditions. Undoped cubic phase LLZO is obtained after calcination at 700–800 °C between 0 and 4 h, and the NAP process is easily extended to Ta-doped LLZO. To elucidate the general formation mechanism of nanosized LLZO in the NAP combustion synthesis, scanning transmission electron microscopy is used to perform energy dispersive X-ray and electron energy loss spectral imaging. The results show that in situ formation of a carbonaceous foam during combustion physically segregates pockets of reagents and is responsible for maintaining the small particle size of the as-synthesized material during combustion and crystallization. The room temperature ionic conductivity of nanosized Ta-doped LLZO synthesized by using the NAP method was studied under various sintering conditions, with ionic conductivities between 0.24 and 0.67 mS cm–1, activation energies between 0.34 and 0.42 eV, and relative densities in excess of 90% obtained by sintering at 1100 °C for between 6 and 15 h.

Facile Synthesis of Al-stabilized Lithium Garnets by a Solution-Combustion Technique for All Solid-state Batteries
Pavan Badami, Stefan Smetaczek, Andreas Limbeck, Daniel Rettenwander, Candace K. Chan and Arunachala Nadar Mada Kannan
Mater. Adv. 2021, 2, 5181-5188
Garnet-type solid electrolytes with cubic modification (c-LLZO, Li7La3Zr2O12) are considered to be one of the most promising candidates for SSLBs with desirable properties such as high ionic conductivity (about 1 mS cm−1) at room temperature, a wide electrochemical operational window, and good stability against reduction by Li metal. The synthesis and processing of garnets through conventional wet-chemical, solid-state reaction and nitrate-combustion approaches often requires one or more of the following processing conditions (energy intensive milling steps, multiple and long periods of calcination) to attain a conductive cubic phase making synthesis time intensive. Herein, we report a facile fuel-assisted solution combustion method using carbohydrazide–nitrate mixtures to synthesize cubic-Li6.28Al0.24La3Zr2O12 (Al-LLZO); compared to other nitrate-combustion approaches, utilizing a nitrogen containing fuel source (CH6N4O) offers drastic reduction in the synthesis duration at relatively low temperatures. Selection of the right fuel to oxidizer ratio and annealing conditions is found to be critical for attaining phase purity and particle growth size of LLZO powders. Cubic phase Al-LLZO with a particle size of up to ∼200 nm was attained at temperatures as low as 800 °C upon calcining the as-combusted powders for 4 h. The green pellets attained high relative densities of 90–92% and ionic conductivities up to 0.45 mS cm−1 at low sintering conditions of 1100 °C for 6 h compared to longer sintering duration (∼10–24 h) for LLZO prepared with a common solid-state reaction or wet chemical methods using conventional pressure-less sintering methods. Sintered pellets exhibited a low activation energy of 0.29 eV likely due to the low grain boundary resistance. Synthesizing sub-micron sized Al-LLZO powders through low-cost facile synthesis approaches is of great importance in the fabrication of composite electrolytes and catholytes.