Photoelectrochemistry and Photocatalysis
Semiconductor Photoelectrochemistry
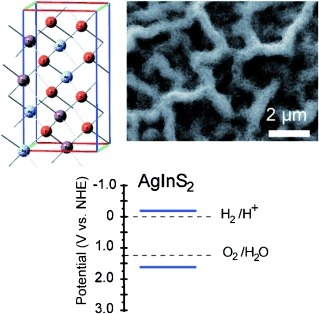
Structural and Photoelectrochemical Evaluation of Nanotextured Sn-Doped AgInS2 Films Prepared by Spray Pyrolysis
Qian Cheng, Xihong Peng, Candace K. Chan
ChemSusChem 2013, 6, 102-109
Spray pyrolysis was used to prepare films of AgInS2 (AIS) with and without Sn as an extrinsic dopant. The photoelectrochemical performance of these films was evaluated after annealing under a N2 or S atmosphere with different amounts of the Sn dopant. DFT was used to calculate the band structure of AIS and understand the role of Sn doping in the observed properties. All AIS films were n-type, and Sn was found to increase the photocurrent and carrier concentration of AIS with an optimum doping level of x=[Sn]/([Ag]+[In])=0.02, which gave a photocurrent of 4.85 mA cm−2. Above this level, the Sn dopants were detrimental to the photoelectrochemical performance, likely a result of a self-compensating effect and the introduction of a deep acceptor level, which could act as a recombination site for photogenerated carriers.
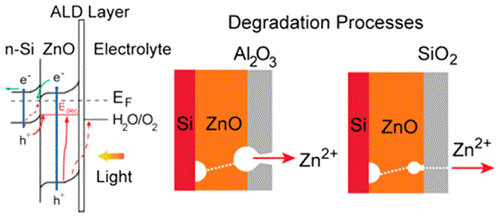
Al2O3 and SiO2 Atomic Layer Deposition Layers on ZnO Photoanodes and Degradation Mechanisms
Qian Cheng, Manpuneet K. Benipal, Qianlang Liu, Xingye Wang, Peter A. Crozier, Candace K. Chan, Robert J. Nemanich
ACS Appl. Mater. Interfaces 2017, 9, 19, 16138–16147
Strategies for protecting unstable semiconductors include the utilization of surface layers composed of thin films deposited using atomic layer deposition (ALD). The protective layer is expected to (1) be stable against reaction with photogenerated holes, (2) prevent direct contact of the unstable semiconductor with the electrolyte, and (3) prevent the migration of ions through the semiconductor/electrolyte interface, while still allowing photogenerated carriers to transport to the interface and participate in the desired redox reactions. Zinc oxide (ZnO) is an attractive photocatalyst material due to its high absorption coefficient and high carrier mobilities. However, ZnO is chemically unstable and undergoes photocorrosion, which limits its use in applications such as in photoelectrochemical cells for water splitting or photocatalytic water purification. This article describes an investigation of the band alignment, electrochemical properties, and interfacial structure of ZnO coated with Al2O3 and SiO2 ALD layers. The interface electronic properties were determined using in situ X-ray and UV photoemission spectroscopy, and the photochemical response and stability under voltage bias were determined using linear sweep voltammetry and chronoamperometry. The resulting surface structure and degradation processes were identified using atomic force, scanning electron, and transmission electron microscopy. The suite of characterization tools enable the failure mechanisms to be more clearly discerned. The results show that the rapid photocorrosion of ZnO thin films is only slightly slowed by use of an Al2O3 ALD coating. A 4 nm SiO2 layer proved to be more effective, but its protection capability could be affected by the diffusion of ions from the electrolyte.
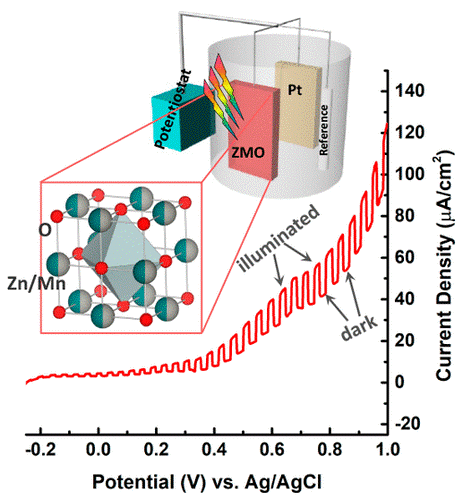
ZnxMn1–xO Solid Solutions in the Rocksalt Structure: Optical, Charge Transport, and Photoelectrochemical Properties
Venkata S. Bhadram, Qian Cheng, Candace K. Chan, Yiqun Liu, Stephan Lany, Kai Landskron, Timothy A. Strobel
ACS Appl. Energy Mater. 2018, 1, 2, 260–266
Theoretical predictions of ZnO:MnO solid solutions (abbreviated here as ZMO) with the rocksalt-type structure suggest improved visible light absorption and suitable band edge positions for the overall water splitting reaction, but experimental efforts to produce such phases are limited by the low solubility of Zn within this structure type. Here, we produce solid solutions of ZnxMn1–xO, with x = 0.5 and 0.3 in the metastable rocksalt phase, using high-pressure and high-temperature (HPHT) techniques. X-ray diffraction and electron microscopy methods were employed to determine the crystal structure, chemical composition, and homogeneity on the submicron scale. The solid solutions exhibit increased optical absorbance in the visible spectral range as compared to those of the parent oxides ZnO and MnO. Our theoretical calculations for ZnxMn1–xO with x = 0.5, 0.25 predict band gaps of 2.53 and 2.98 eV, respectively, with an unusually large band gap bowing. Our calculations also show small effective electron mass for these materials indicating their potential for solar energy applications. Initial photoelectrochemical tests reveal that ZMO solid solutions are suitable for water oxidation and warrant further experimental optimization.
Amorphous Photocatalysts

Preparation of Amorphous and Nanocrystalline Sodium Tantalum Oxide Photocatalysts with Porous Matrix Structure for Overall Water Splitting
Harun Tüysüz and Candace K.Chan
Nano Energy 2013, 2, 116-123
Herein, we report the preparation of a series of surfactant-free nanostructured sodium tantalum oxide using NaTa(OC3H7)6 as a single precursor. The reaction conditions for the novel synthetic method were optimized and the morphology and crystal structure of the prepared materials were investigated by transmission electron microscopy (TEM), X-ray diffraction (XRD), and X-ray photoelectron spectroscopy (XPS). Condensation and polymerization of NaTa(OC3H7)6 under atmospheric pressure gave a porous amorphous structure that could be converted to crystalline NaTaO3 while crystalline Na2Ta2O6 nanocrystals with a 25 nm average particle size could be obtained from a hydrothermal method using NH3 as a base catalyst. In addition, the photocatalytic behaviors of the prepared materials were investigated for overall water splitting into hydrogen and oxygen. Unexpectedly, porous amorphous sodium tantalum oxide showed much better catalytic activity over the crystalline one. The synthesized Na2Ta2O6 nanocrystals also indicated promising activity for overall water splitting without any co-catalyst in comparison to bulk NaTaO3.

High Surface Area, Amorphous Titania with Reactive Ti3+ through a Photo-assisted Synthesis Method for Photocatalytic H2 Generation
Dennis Zywitzki, Hangkun Jing, Harun Tüysüz, Candace K. Chan
J. Mater. Chem. A 2017, 5, 10957-10967
Amorphous titania-based photocatalysts are synthesized using a facile, UV-light mediated method and evaluated as photocatalysts for hydrogen evolution from water/methanol mixtures. The photocatalysts are prepared through the direct injection of a titanium alkoxide precursor into a water/methanol mixture, with subsequent hydrolysis, condensation, and polycondensation to form TiOx(OH)y species under UV-irradiation. The resulting amorphous titania materials exhibit an overall higher hydrogen evolution rate compared to a crystalline TiO2 reference (P25) on a molar basis of the photocatalyst due to their highly porous structure and high surface area (∼500 m2 g−1). The employed titanium alkoxide precursor did not play a major role in affecting the hydrogen evolution rate or the catalyst surface and morphology. A blue coloration, which is associated with the formation of Ti3+ species, was observed in the amorphous titania but not in P25 upon light irradiation and is enabled by the porous and disordered structure of the amorphous photocatalyst. The Ti3+ species are also used to reduce protons to H2 in the absence of light irradiation or reduce Pt2+ to form Pt nanoparticles. These Pt nanoparticles are smaller and better dispersed on the photocatalyst compared to particles prepared using conventional photodeposition, leading to higher H2 evolution rates. The results show that the direct injection method is a facile approach for the preparation of high surface area titania photocatalysts containing Ti3+ species with good photocatalytic activity for the production of H2.

In Situ Total Scattering Experiments of Nucleation and Crystallisation of Tantalum-Based Oxides: From Highly Dilute Solutions via Cluster Formation to Nanoparticles
Ezgi Onur Şahin, Harun Tüysüz, Candace K Chan, Gun-hee Moon, Yitao Dai, Wolfgang Schmidt, Joohyun Lim, Christina Scheu, Claudia Weidenthaler
Nanoscale 2021, 13, 150-162
The exact formation mechanism of tantalum oxides (and in general, metal/mixed metal oxides) from alkoxide precursors is still not fully understood, particularly when forming cluster-like or amorphous materials. The structural evolution of Ta-based oxides was studied in detail using X-ray total scattering experiments along with subsequent pair distribution function (PDF) analyses. Starting from a tantalum alkoxide precursor (Ta2(OEt)10), the formation of hydrolysed TaxOyHz clusters in highly diluted aqueous solution was analysed. From the PDF data, the connectivity and arrangement of TaxOy octahedra in the cluster could be deduced as well as the approximate size of the clusters (<1 nm). Construction of cluster models allowed for identification of common structural motifs in the TaxOyHz clusters, ruling out the formation of chain- or ring-like clusters. More likely, bulky clusters with a high number of corner-sharing octahedra are formed. After separation of the amorphous solid from the liquid, temperature-induced crystallisation processes were monitored via in situ total scattering experiments. Between room temperature and 600 °C, only small rearrangements of the amorphous structure are observed. At about 610 °C, amorphous TaxOyHz transforms directly into crystalline orthorhombic L-Ta2O5 without formation of any crystalline intermediate structures.

Monitoring the Structure Evolution of Titanium Oxide Photocatalysts: From the Molecular Form via the Amorphous State to the Crystalline Phase
Ezgi Onur Şahin, Yitao Dai, Candace K. Chan, Harun Tüysüz, Wolfgang Schmidt, Joohyun Lim, Siyuan Zhang, Christina Scheu, Claudia Weidenthaler
Chem. Eur. J. 2021, 27, 11600-11608
Amorphous TixOy with high surface area has attracted significant interest as photocatalyst with higher activity in ultraviolet (UV) light-induced water splitting applications compared to commercial nanocrystalline TiO2. Under photocatalytic operation conditions, the structure of the molecular titanium alkoxide precursor rearranges upon hydrolysis and leads to higher connectivity of the structure-building units. Structurally ordered domains with sizes smaller than 7 Å form larger aggregates. The experimental scattering data can be explained best with a structure model consisting of an anatase-like core and a distorted shell. Upon exposure to UV light, the white TixOy suspension turns dark corresponding to the reduction of Ti4+ to Ti3+ as confirmed by electron energy loss spectroscopy (EELS). Heat-induced crystallisation was followed by in situ temperature-dependent total scattering experiments. First, ordering in the Ti−O environment takes place upon to 350 °C. Above this temperature, the distorted anatase core starts to grow but the structure obtained at 400 °C is still not fully ordered.
Perovskite Oxide Photocatalysts
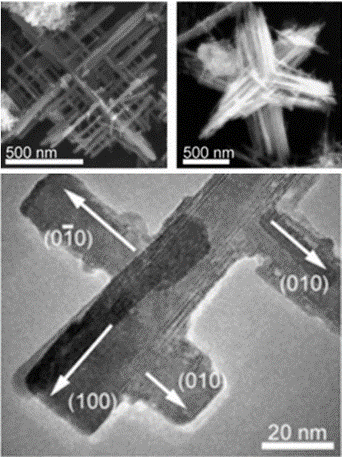
Synthesis of Hyperbranched Perovskite Nanostructures
Ting Yang, Zachary D. Gordon, Candace K Chan
Cryst. Growth Des. 2013, 13, 9, 3901–3907
Complex functional perovskite oxides find use in a wide range of applications as dielectric, electronic, photocatalytic, optical, and ion-conducting materials. The ability to synthesize perovskites in hierarchical structures can allow for new properties or phenomena not observable in bulk morphologies. Herein we report on the solution-phase synthesis of cubic perovskite potassium lanthanum titanate into orthogonal nanostructures with hyperbranched and hexapod morphology through a facile hydrothermal synthesis without using a template, catalyst, substrate, or structure-directing agent. A systematic study of the reaction conditions and role of precursors was performed and a growth mechanism based on homogeneous dissolution–precipitation was determined.
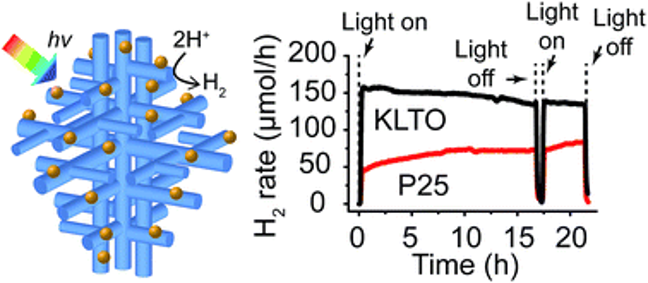
Hyperbranched Potassium Lanthanum Titanate Perovskite Photocatalysts for Hydrogen Generation
Tobias Grewe, Ting Yang, Harun Tüysüz, Candace K. Chan
J. Mater. Chem. A 2016,4, 2837-2841
Semiconductors with hierarchical nanostructured morphologies may be promising as high surface area photocatalysts for producing hydrogen from water. However, there are few scalable synthesis methods that can achieve such morphologies in metal oxide semiconductors such as titanates. Here, hydrothermal methods were used to synthesize nanostructured potassium lanthanum titanate (KLTO) perovskite without using templates or structure-directing agents. The obtained materials were octahedral particles composed of orthogonal hyperbranched nanowires, a morphology that is usually obtained using catalyst-mediated vapor phase methods. Several fundamental materials properties of KLTO were determined for the first time, including the bandgap (3.3 eV), semiconductor type (n-type), flat band potential, and conduction band maximum (−0.265 V and −0.835 V vs. NHE, respectively). The KLTO hyperbranched structures were also investigated as UV-photocatalysts for H2 production and displayed higher activities than P25 TiO2 and KLTO nanoparticles. The H2 production rate for KLTO decorated with 1 wt% Pt using thermal decomposition of K2PtCl4 reached ca. 2.5 mmol h−1 and was stable for 20 h of irradiation.

Synthesis of TiO2 Nanosheet Photocatalysts from Exfoliation of TiS2 and Hydrothermal Treatment
Hangkun Jing, Qian Cheng, J Mark Weller, Ximo S. Chu, Qing Hua Wang, Candace K. Chan
J. Mater. Res. 2018, 33, 3540-3548
TiO2 nanomaterials with platelet or nanosheet morphologies can offer improved properties for photocatalytic applications, but established methods to produce them typically require structure-directing agents since anatase-phase TiO2 does not have a layered structure. In the present work, the preparation of TiO2 nanosheets by the chemical oxidation of TiS2 nanosheets is demonstrated. Electrochemical exfoliation of bulk TiS2 into TiS2 nanosheets, followed by the hydrothermal treatment at 180 °C for 14 h is performed. The results show that polycrystalline TiO2 nanosheets with the anatase structure are formed, and that the nanosheet morphology can still be maintained after the hydrothermal treatment. The TiO2 nanosheets show good photocatalytic activity for the degradation of methylene blue, but the performance is negatively affected by the residual carbon black that was needed in the TiS2 electrode to enable electrochemical exfoliation. These results show that conversion of TiS2 nanosheets to TiO2 nanosheets is a promising synthetic strategy but highlights how the interfacial properties of the obtained materials could be affected by ancillary components in the preparation method.
Mineral-Inspired Catalysts
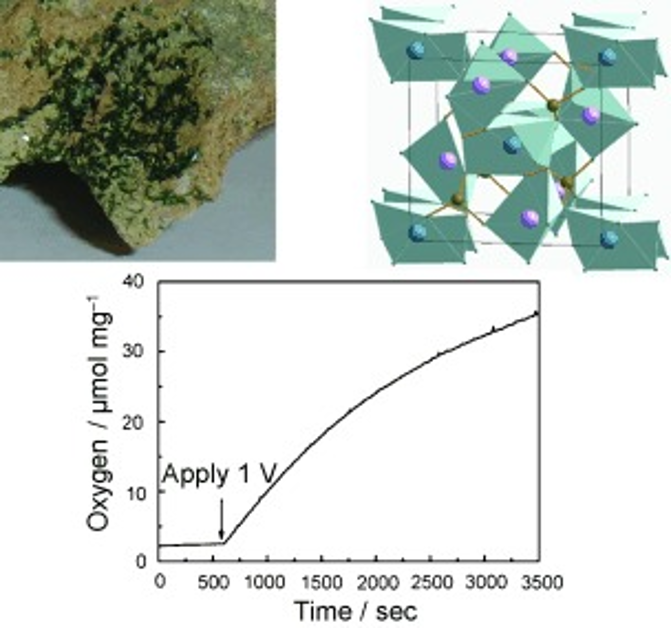
Electrochemical and Photoelectrochemical Properties of the Copper Hydroxyphosphate Mineral Libethenite
ChemElectroChem 2014, 1, 663-672
Man Li, Qian Cheng, Reed M. Wittman, Xihong Peng, Candace K. Chan
There has been much interest recently in the discovery of new, non-oxide materials for photoelectrochemical applications. The copper hydroxyphosphate mineral libethenite, Cu2(OH)PO4 (CHP), which has a Jahn–Teller distorted structure and d9 electron configuration, has recently been reported to display photocatalytic activity. To better understand the properties of this material, a detailed investigation of the relevant fundamental characteristics such as the flatband potential, conduction type, and band diagram was performed using electrochemical and photoelectrochemical methods on CHP thin films deposited onto fluorine-doped tin oxide (FTO) substrates. Density functional theory was used to calculate the band structure, effective mass of electrons and holes, and vacancy formation energies in CHP. CHP was found to be active for electrochemical water oxidation, as confirmed by quantitative O2 measurements. Possible factors behind the low photocurrents observed in linear scanning voltammetry are discussed. Better understanding of this material may lead to the development of improved catalysts and photocatalysts from natural mineral sources.
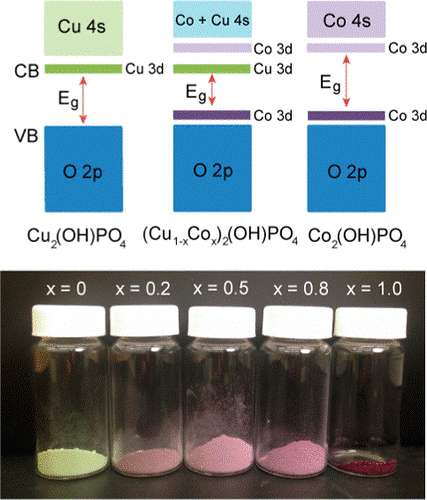
Investigation of the Optical Absorbance, Electronic, and Photocatalytic Properties of (Cu1–xCox)2(OH)PO4 Solid Solutions
Xihong Peng, Man Li, Candace K. Chan
J. Phys. Chem. C 2015, 119, 9, 4684–4693
Transition metal hydroxyl phosphate compounds have attracted recent attention for catalytic and magnetic applications. Here, we present a detailed analysis on the properties of (Cu1–xCox)2(OH)PO4 (0 ≤ x ≤ 1) compounds based on the mineral libethenite. Powders were synthesized using hydrothermal methods, and the photocatalytic activity was evaluated with an Fe3+/Fe2+ redox couple. Introduction of small fractions of Co to Cu2(OH)PO4 increased the photocurrent generation, but greater Co substitution caused it to decrease, with Co2(OH)PO4 showing the lowest photocurrent. The electronic band structure and density of states (DOS) were investigated using standard density functional theory (DFT) and hybrid functional methods. Hybrid DFT provided a better description of the electronic properties, especially the localized Cu and Co d electrons, in good agreement with the experimentally observed band gaps. The addition of Co to Cu2(OH)PO4 led to formation of bands within the band gap arising from Co 3d orbitals, which lowered the band gap <3 eV and changed the band-gap transition from a ligand-to-metal charge transfer (LMCT) to a metal-to-metal charge transfer (MMCT). However, higher concentrations of Co were detrimental to photocurrent generation as a result of the formation of a 3.7 eV MMCT and other electronic factors that could hinder charge separation.
Cobalt Oxide Electrocatalysts

Dual‐Templated Cobalt Oxide for Photochemical Water Oxidation
Xiaohui Deng, Hans‐Josef Bongard, Candace K. Chan, Harun Tüysüz
ChemSusChem 2016, 9, 409-415
Mesoporous Co3O4 was prepared using a dual templating approach whereby mesopores inside SiO2 nanospheres, as well as the void spaces between the nanospheres, were used as templates. The effect of calcination temperature on the crystallinity, morphology, and textural parameters of the Co3O4 replica was investigated. The catalytic activity of Co3O4 for photochemical water oxidation in a [Ru(bpy)3]2+[S2O8]2− system was evaluated. The Co3O4 replica calcined at the lowest temperature (150 °C) exhibited the best performance as a result of the unique nanostructure and high surface area arising from the dual templating. The performance of Co3O4 with highest surface area was further examined in electrochemical water oxidation. Superior activity over high temperature counterpart and decent stability was observed. Furthermore, CoO with identical morphology was prepared from Co3O4 using an ethanol reduction method and a higher turnover-frequency number for photochemical water oxidation was obtained.
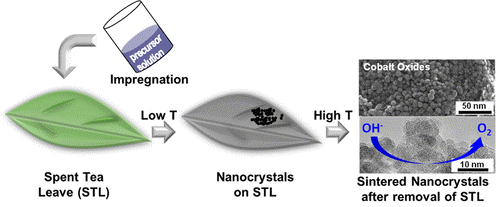
Spent Tea Leaf Templating of Cobalt-Based Mixed Oxide Nanocrystals for Water Oxidation
Xiaohui Deng, Candace K. Chan, Harun Tüysüz
ACS Appl. Mater. Interfaces 2016, 8, 47, 32488–32495
The facile synthesis of nanostructured cobalt oxides using spent tea leaves as a hard template is reported. Following an impregnation–calcination and template removal pathway, sheetlike structures containing nanosized crystallites of Co3O4 are obtained. Co3O4 incorporated with Cu, Ni, Fe, and Mn (M/Co = 1/8 atomic ratio) are also prepared, and the materials are thoroughly characterized using X-ray diffraction, electron microscopy, and N2 sorption. The method is applicable to several commercial tea leaves and is successfully scaled up to prepare over 7 g of Co3O4 with the same nanostructure. The oxides are then tested for electrochemical water oxidation, and Cu, Ni, and Fe incorporations show beneficial effect on the catalytic activity of Co3O4, achieving performance comparable to levels from benchmark electrocatalysts. These data suggest that tea leaf templating can be utilized as a facile and promising approach to prepare nanostructured functional catalyst.
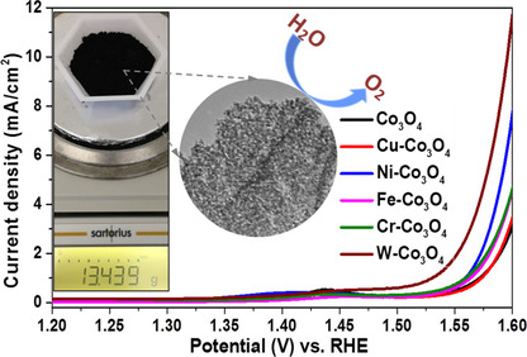
Coffee‐Waste Templating of Metal Ion‐Substituted Cobalt Oxides for the Oxygen Evolution Reaction
Mingquan Yu, Candace K. Chan, Harun Tüysüz
ChemSusChem 2018, 11, 605-611
A facile and scalable method using coffee waste grounds as a hard template has been developed to fabricate nanostructured Co3O4 for the oxygen evolution reaction (OER). Co3O4 incorporating metals with different valences (M/Co=1:4; M=Cu, Ni, Fe, Cr, and W) were also prepared with similar sheet-like structures comprising nanosized crystallites. After detailed characterization by X-ray diffraction, electron microscopy, and nitrogen sorption, the oxides were employed as OER electrocatalysts. Substitution of octahedral and tetrahedral sites of the spinel structure with divalent and trivalent transition metals (Cu, Ni, Fe, and Cr) increased the activity of Co3O4 for the OER, whereas incorporation of hexavalent W led to formation of a second crystal phase and significantly higher electrocatalytic performance. Furthermore, this method is easily scaled up for mass production of Co3O4 with the same nanostructure, which is highly desirable for large-scale application.
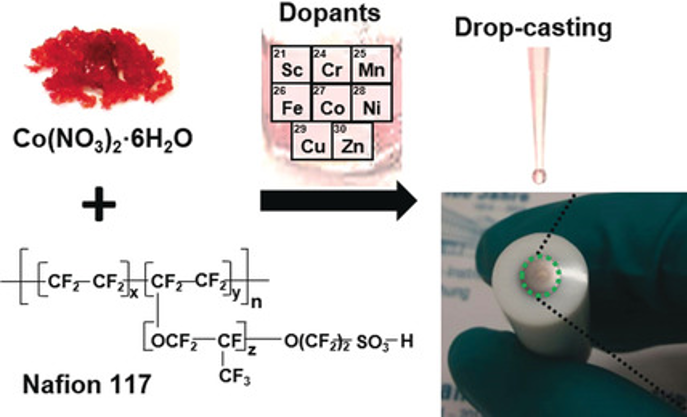
Highly Active Cobalt‐Based Electrocatalysts with Facile Incorporation of Dopants for the Oxygen Evolution Reaction
Gun‐hee Moon, Mingquan Yu, Candace K. Chan, Harun Tüysüz
Angew. Chem. Int. Ed. 2019, 58, 3491
In situ formation of electroactive cobalt species for the oxygen evolution reaction is simply achieved by applying an anodic bias to a commercially available cobalt precursor and Nafion binder mixture coated on a glassy carbon electrode. This preparation does not require energy-intensive materials preparation steps or noble metals, yet a low overpotential of 322 mV at 10.2 mA cm−2 and a high current density of more than 300 mA cm−2 at 1.7 VNHE were obtained in 1 m KOH. An operando electrochemical Raman spectroscopy study confirmed the formation of cobalt oxyhydroxide species and the iron stimulated the equilibrium state between Co3+ and Co4+. The iron present in the alkali electrolyte or ink solution effectively activated the cobalt species, and most of the first row transition metals could also enhance the catalytic performance. The concept presented here is one of the simplest strategies for preparing highly active electrocatalysts and is very flexible for the replacement of cobalt by other transition metals.
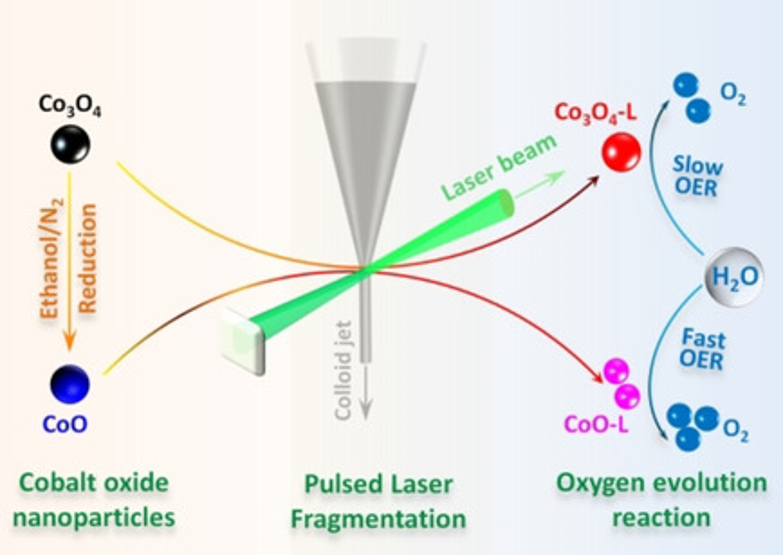
Laser Fragmentation‐Induced Defect‐Rich Cobalt Oxide Nanoparticles for Electrochemical Oxygen Evolution Reaction
Mingquan Yu, Friedrich Waag, Candace K. Chan, Claudia Weidenthaler, Stephan Barcikowski, Harun Tüysüz
ChemSusChem 2020, 13, 520-528
Sub-5 nm cobalt oxide nanoparticles are produced in a flowing water system by pulsed laser fragmentation in liquid (PLFL). Particle fragmentation from 8 nm to 4 nm occurs and is attributed to the oxidation process in water where oxidative species are present and the local temperature is rapidly elevated under laser irradiation. Significantly higher surface area, crystal phase transformation, and formation of structural defects (Co2+ defects and oxygen vacancies) through the PLFL process are evidenced by detailed structural characterizations by nitrogen physisorption, electron microscopy, synchrotron X-ray diffraction, and X-ray photoelectron spectroscopy. When employed as electrocatalysts for the oxygen evolution reaction under alkaline conditions, the fragmented cobalt oxides exhibit superior catalytic activity over pristine and nanocast cobalt oxides, delivering a current density of 10 mA cm−2 at 369 mV and a Tafel slope of 46 mV dec−1, which is attributed to a larger exposed active surface area, the formation of defects, and an increased charge transfer rate. The study provides an effective approach to engineering cobalt oxide nanostructures in a flowing water system, which shows great potential for sustainable production of active cobalt catalysts.