Nanomaterials for Water Treatment
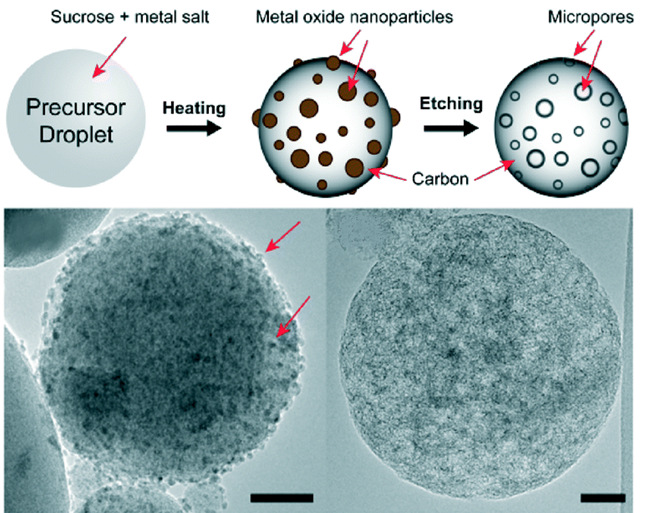
Carbon Nanosphere Adsorbents for Removal of Arsenate and Selenate from Water
Man Li, Chengwei Wang, Michael J. O’Connell, Candace K Chan
Environ. Sci.: Nano, 2015, 2, 245-250
Porous carbon nanospheres prepared using spray pyrolysis were evaluated as adsorbents for removal of arsenate and selenate in de-ionized (DI), canal, and well waters. The carbon nanospheres displayed good binding to both metals in DI water and outperformed commercial activated carbons for arsenate removal in pH > 8, likely due to the presence of basic surface functional groups, high surface-to-volume ratio, and suitable micropores formed during the synthesis.
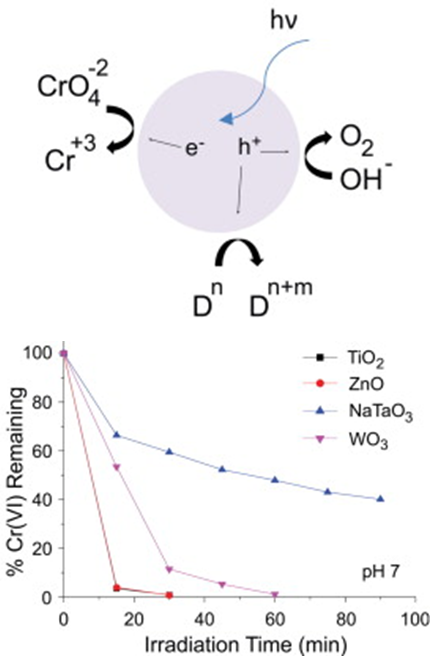
Hexavalent Chromium Removal using Metal Oxide Photocatalysts
Qian Cheng, Chengwei Wang, Kyle Doudrick, Candace K. Chan
Appl. Catal. B: Environ. 2015, 176-177, 740-749
Photocatalysis is an attractive treatment method for removing hexavalent chromium (Cr(VI)) from water. Thus far, photocatalytic reduction of Cr(VI) has been investigated mostly using TiO2 photocatalysts in acidic water solutions. Here we investigate Cr(VI) removal using zinc oxide (ZnO), tungsten trioxide (WO3), and sodium tantalate (NaTaO3), metal oxides that display good activity for other photocatalytic reactions such as water splitting, as well as titanium oxide (TiO2, Evonik P90). The efficiency for Cr(VI) removal using these photocatalysts was investigated in synthetic neutral and alkaline water, as well as in cooling tower blowdown water. The effect of several additives used in water treatment processes on the Cr(VI) removal rate was also studied. For NaTaO3, citric acid was found to have a detrimental effect to Cr(VI) removal, while sodium formate, ammonium chloride, and sodium sulfite were beneficial. While sulfite alone could chemically reduce Cr(VI), sulfite in combination with a photocatalyst resulted in faster and complete removal of Cr(VI) in 10 min using a SO32−/Cr(VI) ratio >35 in pH ∼ 8 solutions. NaTaO3 was found to display the highest Cr(VI) removal rates on a photon basis at pH 3 and in the presence of sodium sulfite, while ZnO and TiO2 showed the best performance in pH 7 and cooling tower blowdown water.
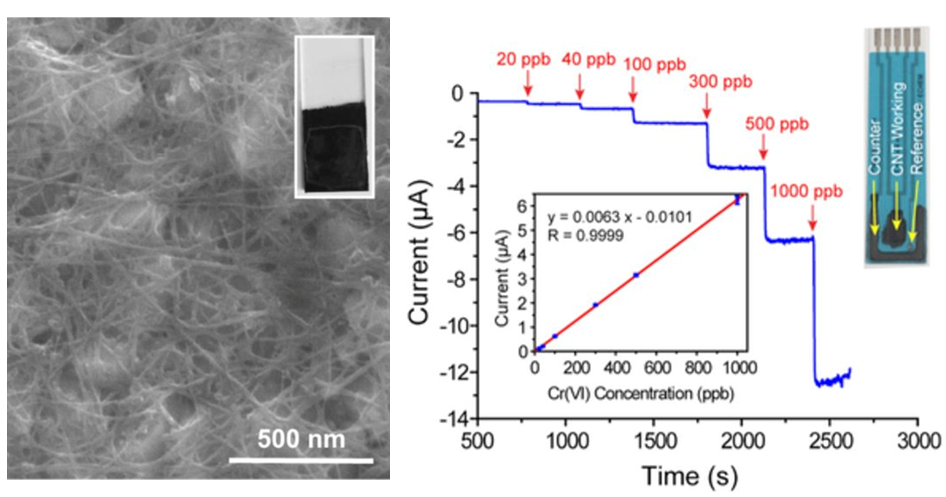
Carbon Nanotube–based Electrodes for Detection of Low–ppb Level Hexavalent Chromium Using Amperometry
Chengwei Wang, Candace K. Chan
ECS J. Solid State Sci. Technol. 2016, 5, M3026
Carbon nanotube (CNT)-based electrodes, prepared using a printable technique, were investigated for the electrochemical detection of hexavalent chromium (Cr(VI)). CNT pastes were coated onto paper substrates and commercially available screen-printed electrodes and used as amperometric sensors for Cr(VI). The CNT electrodes showed electrochemical current responses as high as 500 nA/ppb Cr(VI) and limit of detection as low as 5 ppb when a large area electrode was used. The CNT-modified, screen-printed electrodes showed good selectivity to Cr(VI) and were effective for quantifying the Cr(VI) levels in cooling tower blowdown water. A selective H2O2 reduction technique was also applied to Cr(VI) detection and integrated into amperometric detection in a flow cell. These studies show that CNT-based electrodes can be promising for field applications and real-time monitoring of low-level Cr(VI) in power plant waters.
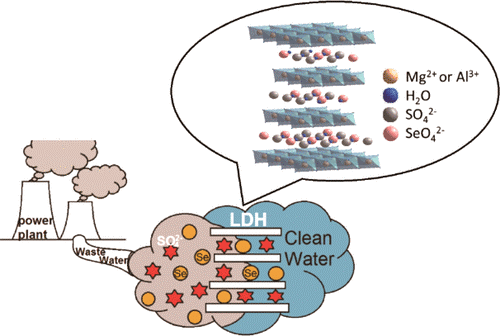
Selenium Removal from Sulfate-Containing Groundwater Using Granular Layered Double Hydroxide Materials
Man Li, Lisa M. Farmen, Candace K. Chan
Ind. Eng. Chem. Res. 2017, 56, 9, 2458–2465
Due to recent changes in regulatory discharge limits, selenium removal from electric utility wastewaters is becoming an important issue. In this work, Mg–Al–CO3 layered double hydroxide (LDH) in granular form was evaluated for treatment of selenium-containing groundwater in small scale column tests. The LDH material showed good capacity for removing selenate from groundwaters that contained very high levels of sulfate and total dissolved solids. Column tests were investigated using the granular LDH to treat groundwater containing trace levels of selenium <2 ppb. Removal of sulfate using chemical pretreatment of the groundwater resulted in about 3× higher selenium loading onto the granular LDH. The structural changes in the media after exhaustion and analysis of the effluent water from the column test were also studied to better understand the species removed by the LDH. These results show that the LDH is a promising sorbent for removing selenium from wastewaters with high levels of sulfate and background species.

Layered Double Hydroxide/Chitosan Nanocomposite Beads as Sorbents for Selenium Oxoanions
Man Li, Andrew Dopilka, Andrea N. Kraetz, Hangkun Jing, Candace K. Chan
Ind. Eng. Chem. Res. 2018, 57, 14, 4978–4987
Layered double hydroxide (LDH) nanoparticles are effective sorbents for selenium oxoanions but must be fabricated in a suitable fashion for implementation in water treatment applications using packed columns. In this work, we demonstrate the preparation of nanocomposite beads prepared from Mg–Al–CO3 LDH nanoparticles and chitosan, a sustainable and biodegradable biopolymer. The synthesis of the nanocomposite beads is achieved by direct mixing or in situ synthesis of the LDH nanoparticles into the chitosan matrix. The effect of the preparation route on the nanocomposite structure, maximum loading of LDH in the composite, removal kinetics, and the maximum sorption capabilities for selenate and selenite oxoanions are studied and compared to LDH nanopowders and granular media. The results indicate that the in situ synthesis of LDH inside the beads leads to several favorable characteristics such as a higher mass loading of LDH and better dispersion of the nanoparticles while displaying good selenium removal over a wide pH range, superior sorption capacities to the nanopowder, and sorption kinetics similar to those of the granulated media. The maximum adsorption capacities for the nanocomposite beads from Langmuir isotherms were 17 mg/g for Se(IV) and ∼12 mg/g for Se(VI) with respect to the mass of LDH, which is higher than reported capacities obtained in chitosan beads embedded with other nanocrystalline metal oxide fillers. These results show that the LDH/chitosan nanocomposite beads are promising alternatives to granulated media for selenium removal and sheds light on how best to design and fabricate high performance and sustainable nanoenabled sorbents for water treatment applications.

Layered Double Hydroxide Sorbents for Removal of Selenium from Power Plant Wastewaters
Man Li, Tanzil Chowdhury, Andrea N. Kraetz, Hangkun Jing, Andrew Dopilka, Lisa M. Farmen, Shahnawaz Sinha, Candace K. Chan
ChemEngineering 2019, 3, 20
Selenium is an essential trace element but is increasingly becoming a contaminant of concern in the electric power industry due to the challenges of removing solubilized selenate anions, particularly in the presence of sulfate. In this work, we evaluate granulated layered double hydroxide (LDH) materials as sorbents for selenium removal from wastewaters obtained from a natural gas power plant with the aim to elucidate the effect of competing ions on the sorption capacities for selenium removal. We first present jar test data, followed by small-scale column testing in 0.43 inch (1.1 cm) and 2 inch (5.08 cm) diameter testbed columns for the treatment of as-obtained cooling tower blowdown waters and plant wastewaters. Finally, we present field results from a pilot-scale study evaluating the LDH media for treatment of cooling tower blowdown water. We find that despite the high levels of total dissolved solids and competing sulfate ions, the selenium oxoanions and other regulated metals such as chromium and arsenic are successfully removed using LDH media without needing any pre-treatment or pH adjustment of the wastewater.
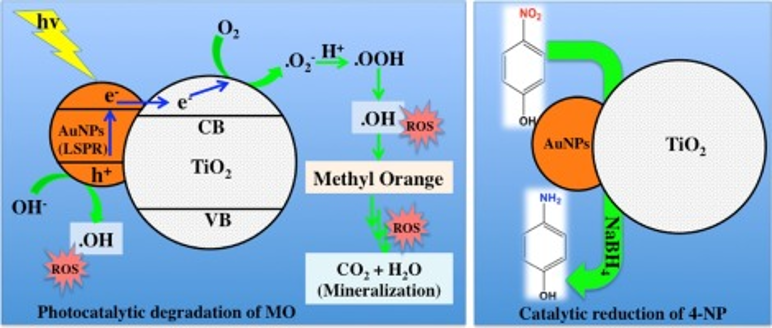
Fullerene Stabilized Gold Nanoparticles Supported on Titanium Dioxide for Enhanced Photocatalytic Degradation of Methyl Orange and Catalytic Reduction of 4-nitrophenol
Md Tariqul Islam, Hangkun Jing, Ting Yang, Emmanuel Zubia, Alan G. Goos, Ricardo A. Bernal, Cristian E. Botez, Mahesh Narayan, Candace K. Chan, Juan C. Noveron
J. Environ. Chem. Eng. 2018, 4, 3827-3836
A facile method for the synthesis of gold nanoparticles (AuNPs) supported on TiO2 is reported. The average size of the TiO2 supported AuNPs was found to be about 8 nm, which was measured by the transmission electron microscopy. The TiO2 supported AuNPs exhibited enhanced photocatalytic degradation of methyl orange (MO) and catalytic reduction of 4-nitrophenol (4-NP) in water. Both the photocatalytic degradation of MO and the catalytic reduction of 4-NP were influenced by the size and the percent AuNPs loading. Compared to the pristine TiO2 the nanocomposite having 4.76 wt% AuNPs showed about twice and 132 times faster activity in the photodegradation of MO and the reduction of 4-NP, respectively The cyclic stability of the nanocomposite was examined for ten cycles and it was found that the catalyst is fairly active throughout the cycles. Further, the photocatalytic generation of hydroxyl radical was confirmed through the terephthalic acid photoluminescence tests.

Synthesis of TiO2 Nanosheet Photocatalysts from Exfoliation of TiS2 and Hydrothermal Treatment
Hangkun Jing, Qian Cheng, J. Mark Weller, Ximo S. Chu, Qing Hua Wang, Candace K. Chan
J. Mater. Res. 2018, 33, 3540-3548
TiO2 nanomaterials with platelet or nanosheet morphologies can offer improved properties for photocatalytic applications, but established methods to produce them typically require structure-directing agents since anatase-phase TiO2 does not have a layered structure. In the present work, the preparation of TiO2 nanosheets by the chemical oxidation of TiS2 nanosheets is demonstrated. Electrochemical exfoliation of bulk TiS2 into TiS2 nanosheets, followed by the hydrothermal treatment at 180 °C for 14 h is performed. The results show that polycrystalline TiO2 nanosheets with the anatase structure are formed, and that the nanosheet morphology can still be maintained after the hydrothermal treatment. The TiO2 nanosheets show good photocatalytic activity for the degradation of methylene blue, but the performance is negatively affected by the residual carbon black that was needed in the TiS2 electrode to enable electrochemical exfoliation. These results show that conversion of TiS2 nanosheets to TiO2 nanosheets is a promising synthetic strategy but highlights how the interfacial properties of the obtained materials could be affected by ancillary components in the preparation method.

Titanium Dioxide–Layered Double Hydroxide Composite Material for Adsorption–Photocatalysis of Water Pollutants
Min-Jeong Suh, Yi Shen, Candace K. Chan, Jae-Hong Kim
Langmuir 2019, 35, 26, 8699–8708
Although adsorption has gained favor among numerous water treatment technologies as an effective pollutant removal method, its application is often hindered by challenges with its resource- and energy-intensive regeneration procedure once the available adsorption sites are exhausted. Herein, we present adsorption–photocatalysis composite materials combining layered double hydroxides (LDHs) and titanium dioxide (TiO2) for water treatment. Incorporation of the photocatalyst into the material opens opportunities to harness light from the sun or lamps for oxidative degradation of the adsorbed contaminants on the material surface, to free adsorption sites for material reuse. In addition to allowing photocatalytic regeneration, the addition of TiO2 to colloidal suspensions of delaminated LDH enabled the formation of TiO2–LDH composites with far superior adsorptive performances compared to their parent LDH compounds. During the material synthesis, positively charged LDH layers and negatively charged TiO2 particles combine through electrostatic attraction to yield composites with dramatically enhanced adsorption capacities toward model contaminants, methyl orange and 2,4-dichlorophenoxyacetic acid, by 16.0 and 76.7 times, respectively. Combining delaminated LDH with TiO2 allowed us to maximize the exposure of positively charged surfaces to the contaminants, in a form that can be used as a solid adsorbent. After regeneration, the material regained up to 92% of its adsorption efficiency toward model contaminants. In light of our findings showing significantly different kinetics of adsorption and photocatalytic regeneration, we propose a new scheme to utilize adsorption–photocatalysis systems, in which the two processes are separated to better utilize their unique strengths.
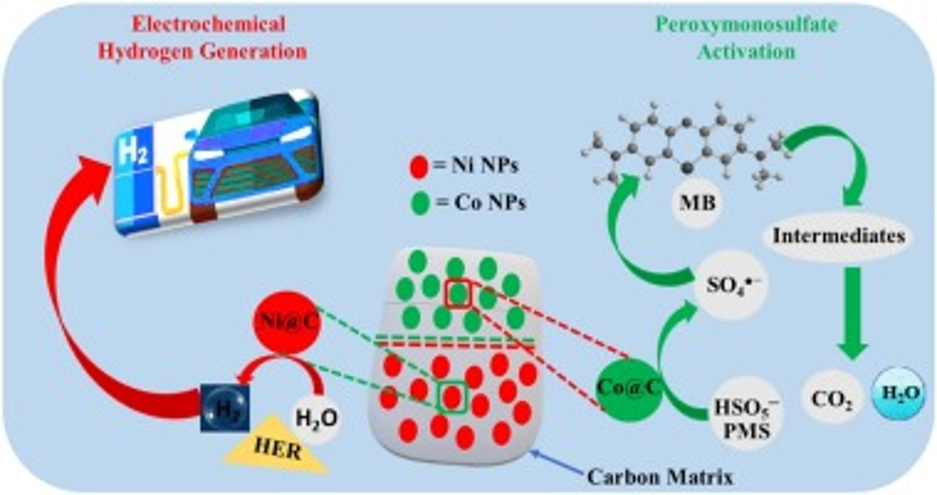
Metal-Organic Frameworks-Derived Multifunctional Carbon Encapsulated Metallic Nanocatalysts for Catalytic Peroxymonosulfate Activation and Electrochemical Hydrogen Generation
Md Ariful Ahsan, Alain R. Puente Santiago, Aruna Narayanan Nair, J. Mark Weller, Mohammed F. Sanad, Delia J. Valles-Rosales, Candace K. Chan, Sreeprasad Sreenivasan, Juan C. Noveron
Mol. Catal. 2020, 498, 111241
Synthesis of high-efficiency metal catalysts and their application in catalyzing critical chemical processes holds the key to the sustainable supply of water and energy. However, reaction-induced atomistic modifications of nanoclusters often result in reduced stability and efficacy. Herein, we report a highly active and multifunctional transition metal nanocatalysts encapsulated in porous carbon network (M@C where M = Cu, Ni, Fe, Co) prepared by leveraging the sacrificial templating properties of metal-organic frameworks (MOFs). The as-synthesized M@C nanocatalysts were employed for oxidative degradation of organic pollutants and electrocatalytic hydrogen generation. Fenton like catalytic studies revealed that the nanocatalysts were highly active and reusable following the order of Co@C > Fe@C > Cu@C > Ni@C. On the other hand, Ni@C electrocatalyst displayed superior activity towards hydrogen evolution reaction as compared to others, delivering a low onset potential of 61 mV, Tafel slope of 82 mV/dec and an overpotential of 286 mV at 10 mA‧cm−2. The activity was essentially unchanged even after 500 cycles, suggesting the long‐term stability under acidic conditions. The impressive multifunctional catalytic performances of M@C nanocatalysts are attributed to their unique porous carbon matrix doped by transition metal nanoparticles which provide a large number of interconnected catalytically active sites.
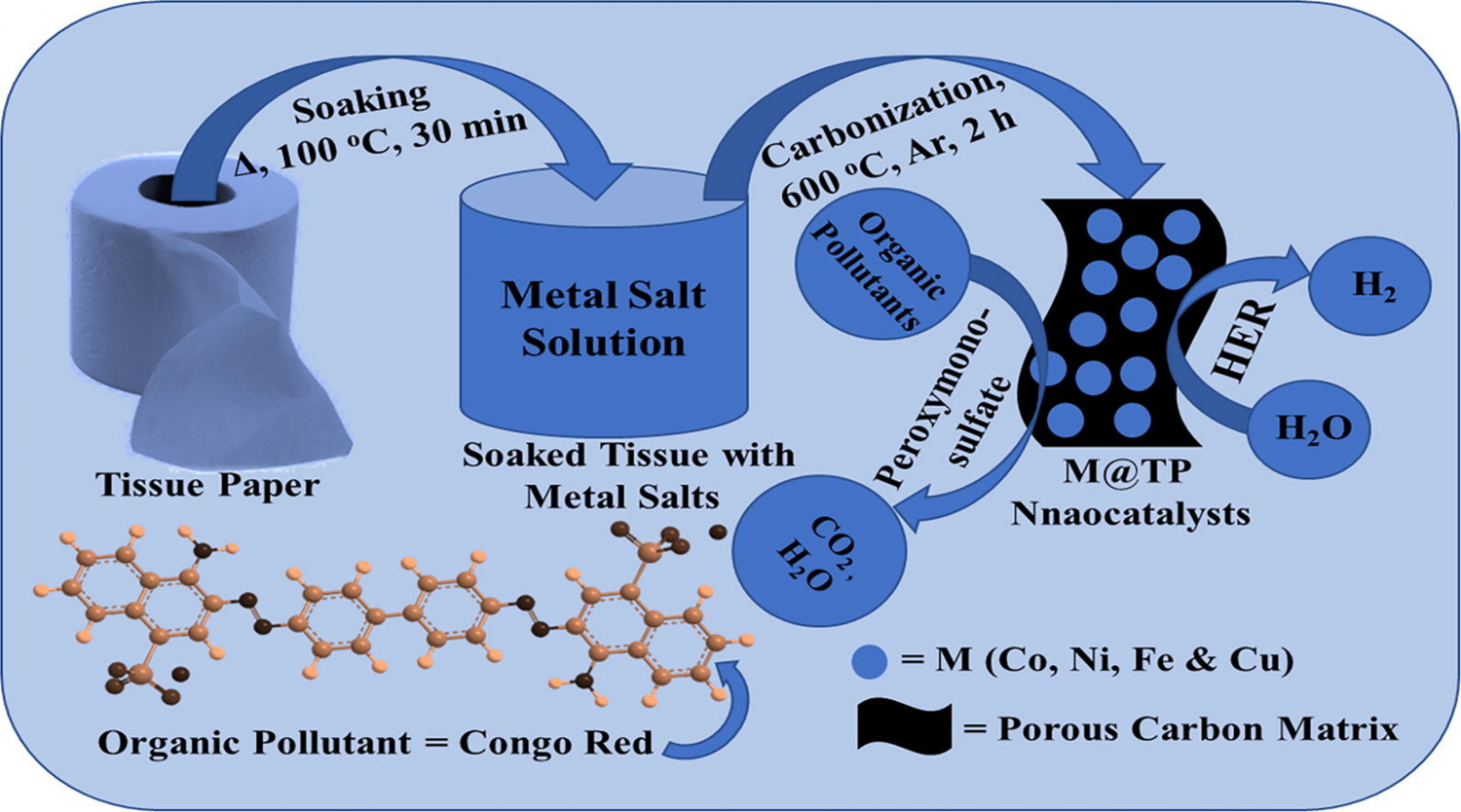
Tissue Paper-Derived Porous Carbon Encapsulated Transition Metal Nanoparticles as Advanced Non-Precious Catalysts: Carbon-Shell Influence on the Electrocatalytic Behaviour
Md Ariful Ahsan, Alain R. Puente Santiago, Mohamed F. Sanad, J. Mark Weller, Olivia Fernandez-Delgado, Luis A. Barrera, Viridiana Maturano-Rojas, Bonifacio Alvarado-Tenorio, Candace K. Chan, Juan C. Noveron
J. Colloid Interface Sci. 2021, 581, 905-918
Porous carbon encapsulated non-precious metal nanocatalysts have recently opened the ways towards the development of high-performance water remediation and energy conversion technologies. Herein, we report a facile, scalable and green synthetic methodology to fabricate porous carbon encapsulated transition metal nanocatalysts (M@TP: M = Cu, Ni, Fe and Co) using commercial tissue paper. The morphology, crystalline structure, chemical composition and textural properties of the M@TP nanocatalysts were thoroughly characterized. The catalytic activity of the M@TP nanocatalysts was investigated for the degradation of Congo red (CR) via peroxymonosulfate activation. Co@TP-6 was found to be the most active catalyst allowing 97.68% degradation in 30 min with a higher rate constant of 0.109 min−1. The nanocatalysts also displayed a carbon shell thickness-dependent electrocatalytic hydrogen evolution reaction (HER) activity, most likely due to the shielding effect of the carbon layers over the electron transfer (ET) processes at the metal core/carbon interfaces. Remarkably, the Ni@TP-6 electrocatalyst, with the smaller carbon shell thickness, showed the best electrocatalytic performance. They delivered an ultralow onset potential of −30 mV vs RHE, an overpotential of 105 mV at a current density of 10 mA·cm−2 and an excellent electrochemical stability to keep the 92% of the initial current applied after 25000 s, which is comparable with the HER activity of the state-of-the-art Ni-based catalysts.

Emerging Opportunities for Nanotechnology to Enhance Water Security
Pedro J.J. Alvarez, Candace K. Chan, Menachem Elimelech, Naomi J. Halas, Dino Villagrán
Nature Nanotech. 2018, 13, 634-641
No other resource is as necessary for life as water, and providing it universally in a safe, reliable and affordable manner is one of the greatest challenges of the twenty-first century. Here, we consider new opportunities and approaches for the application of nanotechnology to enhance the efficiency and affordability of water treatment and wastewater reuse. Potential development and implementation barriers are discussed along with research needs to overcome them and enhance water security.